Actin Filaments: Essential Components of the Cytoskeleton
- I. Introduction
- II. Structure of Actin Filaments
- III. Functions of Actin Filaments
- IV. Regulation of Actin Filaments
- V. Regulatory Mechanisms
- VI. Actin Filaments in Specialized Cells
- VII. Actin Filaments in Disease
- VIII. Experimental Techniques to Study Actin Filaments
- IX. Actin Filaments in Biotechnology and Therapeutics
- X. Conclusion
- References:
- Image References:
I. Introduction
The cytoskeleton, an intricate network of protein filaments, is fundamental to the structural integrity and functional dynamics of eukaryotic cells. Among its components, actin filaments—also known as microfilaments—play a pivotal role in a myriad of cellular processes, including motility, shape maintenance, and intracellular transport. Composed of globular actin monomers (G-actin) that polymerize to form long, filamentous structures (F-actin), these filaments exhibit a dynamic behavior that is critical for various cellular activities. The ability of actin filaments to rapidly assemble and disassemble enables cells to respond effectively to environmental cues, facilitating processes such as cytokinesis, changes in cell morphology, and muscle contraction. A deeper exploration of actin filaments reveals not only their critical contributions to cellular architecture but also their interactions with other cytoskeletal elements, highlighting their essential role in maintaining overall cellular function and integrity.
A. Overview of the cytoskeleton and its components
The cytoskeleton serves as a critical structural framework within eukaryotic cells, comprising three main types of filamentous networks: actin filaments (microfilaments), intermediate filaments, and microtubules. Each component plays distinct roles in maintaining cellular integrity and facilitating dynamic processes such as motility, division, and intracellular transport. Actin filaments are particularly notable for their versatility; they not only provide structural support but are also instrumental in cell shape changes and locomotion through the formation of structures like lamellapodia. Furthermore, the interplay between the cytoskeletal components is underscored by the concept of biochemical-mechanical feedback loops that enhance cellular adhesion and contractility, reinforcing the actin cytoskeletons significance in these processes (Besser A et al.). The cytoskeleton’s ability to balance stability with flexibility is fundamental to cellular function, allowing the cell to adapt to various environmental stimuli while maintaining its structural integrity (Szabo B et al.).
B. Role of actin filaments in cellular structure and function
Actin filaments play a pivotal role in maintaining cellular structure and facilitating various cellular functions. As the most abundant component of the cytoskeleton, actin filaments contribute to the structural integrity of the cell, shaping its morphology and enabling motility through dynamic remodeling. Their ability to undergo rapid polymerization and depolymerization allows cells to adapt quickly to environmental changes, promoting processes such as cell division and shape changes during migration. Additionally, actin filaments are integral to the mechanical properties of specialized cells, including chondrocytes, where their disruption leads to marked alterations in elasticity and viscoelasticity, thereby underlining their mechanical significance in maintaining cellular functionality (Blanchette et al.). Furthermore, the interplay between actin and other cytoskeletal components, such as intermediate filaments, suggests a coordinated mechanism that regulates the overall cellular response to various stimuli, reinforcing the essential nature of actin filaments within the cytoskeletal framework (Szabo B et al.).
C. Historical discovery and significance of actin filaments
The historical discovery of actin filaments marks a significant milestone in cellular biology, fundamentally altering our understanding of the cytoskeletons architecture and function. Initially identified in muscle tissue, actin was recognized for its role in muscle contraction; however, subsequent studies unveiled its ubiquitous distribution and participation in various cellular processes. For instance, the discovery of actins dynamic properties highlighted its involvement in cell motility and division, essential for organismal development and tissue repair. Key developments in microscopy allowed scientists to visualize actin structures, contributing to the identification of its regulatory components, such as tropomyosin, which modulates actins interactions during muscle contraction, as depicted in . Furthermore, contemporary research emphasizes actins influence on cancer cell behavior, revealing that alterations in actin dynamics can impact tumor growth and metastasis, reinforcing its importance in cellular physiology as substantiated by findings in (Kressirer et al.). Thus, the historical journey of actin filaments underscores their critical role in the cytoskeleton and broader physiological implications.
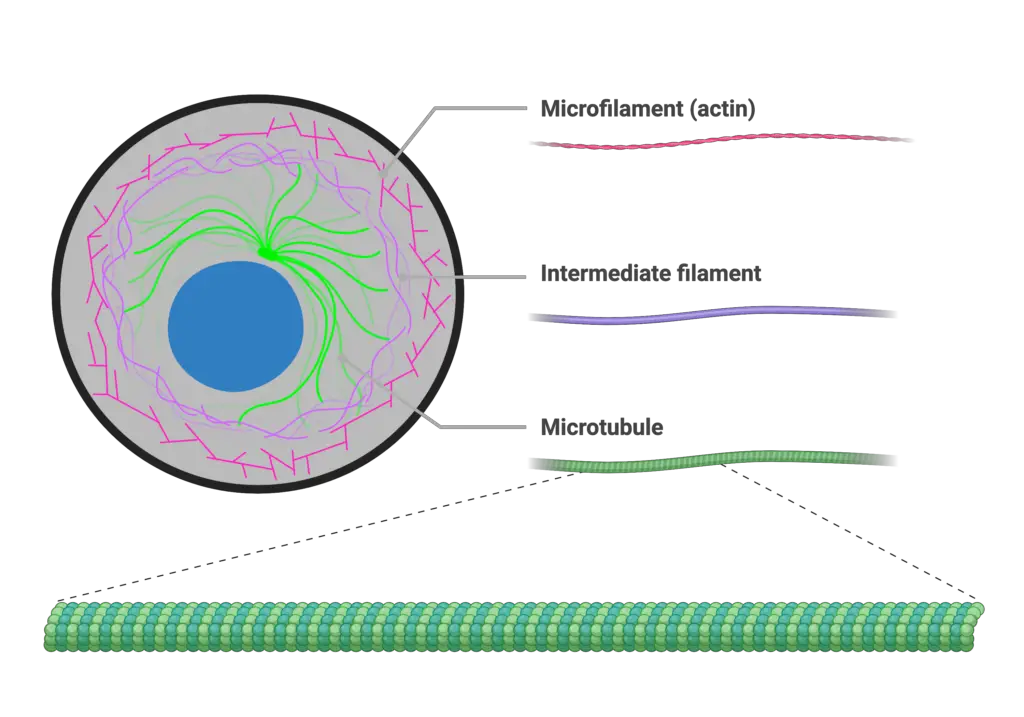
Image1 : Diagram of cellular cytoskeleton components: microfilaments, intermediate filaments, and microtubules.
II. Structure of Actin Filaments
Understanding the structure of actin filaments is integral to comprehending their numerous cellular functions. Actin, a globular protein (G-actin), polymerizes to form long, helical filaments (F-actin) that exhibit dynamic properties essential for cellular integrity and motility. This dynamic nature is characterized by cycles of assembly and disassembly, which are crucial for the maintenance and remodeling of the cytoskeleton. Notably, research indicates that the stability of these filaments is influenced by various actin-binding proteins that either stabilize or disrupt filament formation, as seen in the rapid cytoskeletal disruption caused by the actin assembly inhibitor latrunculin-A (LAT-A) in yeast cells, underscoring the delicate balance within actin dynamics (Adams et al.). Additionally, actin filaments demonstrate a unique structural organization that facilitates their role in various cellular processes, including muscle contraction and intracellular transport, reflecting a complex interplay of forces within the cellular environment (A Lau et al.).
A. Molecular composition of actin
The molecular composition of actin is fundamental to its diverse functional roles within eukaryotic cells, especially as a critical component of the cytoskeleton. Actin exists in two primary forms: globular actin (G-actin), which is a monomeric unit, and filamentous actin (F-actin), which comprises long polymers of G-actin. This polymerization allows for the dynamic nature of actin filaments, as they can rapidly assemble and disassemble in response to cellular signals. Notably, the electrical properties of actin are significant; polymerized actin exhibits lower impedance compared to its globular form, facilitating electrical signaling within cells ((Paladini S et al.)). Furthermore, actins interaction with remodeling proteins, such as cofilin, plays a crucial role in regulating filament dynamics and contributing to inflammatory responses in various conditions ((Xing J et al.)). Thus, understanding the molecular composition of actin provides insight into its essential functions in maintaining cellular architecture and enabling dynamic cellular processes.
| Component | MolecularWeight kDa | Function | Abundance in Cell |
| Actin Monomer (Globular Actin) | 42 | Polymerizes to form filaments | Around 10% of total protein |
| ATP-bound Actin | 42 | Initiates actin filament growth | Variable, depends on cellular conditions |
| ADP-bound Actin | 42 | Represents the filamentous state, less stable | Variable, influenced by ATP hydrolysis |
| Actin Binding Proteins | Varies (5-200) | Regulate polymerization, nucleation, and stabilization | Diverse, related to specific cellular functions |
| Cross-linking Proteins | Varies (10-150) | Form networks and create structural support | Present in varying concentrations |
Molecular Composition of Actin
B. Polymerization process of actin filaments
Actin filaments, essential components of the cytoskeleton, undergo a dynamic polymerization process that is critical for cellular motility and structural integrity. This process begins with the nucleation of actin monomers, driven by the presence of ATP-bound G-actin, which assembles into filamentous F-actin. The polymers growth is characterized by a rapid addition of monomers at the barbed end, while depolymerization occurs at the pointed end, fostering a constant turnover that adapts to cellular requirements ((Risler et al.)). Notably, this actin dynamics underlies various morphological states in cells, such as the transition between non-migrating and migrating states, which hinge on the spatial distribution and dynamics of actin and myosin molecules ((Alt et al.)). Consequently, understanding the mechanisms of actin polymerization not only reveals its role in maintaining cell shape but also elucidates its significance in complex behaviors such as cell migration and division.
C. Structural characteristics of actin filaments
The structural characteristics of actin filaments are foundational to their diverse roles within the cytoskeleton. Composed of globular actin (G-actin) monomers that polymerize to form filamentous actin (F-actin), these filaments exhibit a dynamic nature that is critical for cellular processes such as motility and shape maintenance. The helical arrangement of F-actin contributes to its mechanical properties, allowing it to withstand various cellular stresses while maintaining flexibility. Notably, the interactions between actin filaments and a myriad of regulatory proteins enable precise control over filament assembly and disassembly, as underscored in studies of active networks showing how crosslinkers influence filament dynamics (Farhadi et al.). Furthermore, the intrinsic polarity of actin filaments—characterized by a fast-growing barbed end and a slow-growing pointed end—facilitates directional movement of motor proteins, integrating actins structural attributes with its functional capabilities in cellular contexts (Szabo B et al.).
D. Comparison with other cytoskeletal components
In the landscape of the cytoskeleton, actin filaments are often contrasted with microtubules and intermediate filaments, each contributing distinct functions to cellular architecture and dynamics. While actin filaments are known for their ability to rapidly polymerize and depolymerize, facilitating processes like cell motility and shape changes, microtubules provide structural rigidity and are essential for intracellular transport and mitotic spindle formation. Intermediate filaments, on the other hand, lend mechanical support and stability to cells, anchoring organelles and offering resistance to tensile stress. An intriguing aspect of this comparison is the molecular machinery behind cell motility; as noted in (Risler et al.), actin dynamics underlie various motility mechanisms, while (Barbarese et al.) highlights the interactions of translation factors with the cytoskeleton, emphasizing the importance of actin in localized translation. This synergy among cytoskeletal components underscores their interconnected roles in maintaining cellular homeostasis and facilitating functional diversity.
| Component | Diameter (nm) | Polymerization Speed (nm/s) | Main Functions | Abundance in Cells |
| Actin Filaments | 7 | 10 | Cell shape, motility, division | Highly abundant |
| Microtubules | 25 | 40 | Cell shape, intracellular transport, mitosis | Moderately abundant |
| Intermediate Filaments | 10 | 1 | Structural support, mechanical strength | Variable abundance |
Comparison of Cytoskeletal Components
III. Functions of Actin Filaments
Understanding the functions of actin filaments is crucial for elucidating their role as dynamic components of the cytoskeleton. Actin filaments, also known as microfilaments, are integral to various cellular processes, including maintaining cell shape, facilitating cellular motility, and enabling intracellular transport. The mechanical properties of actin filaments are directly linked to their ability to undergo rapid polymerization and depolymerization, which enables cells to adapt their structure quickly in response to external stimuli ( et al.). Actin dynamics play a fundamental role in processes such as endocytosis and vesicle transport, which are essential for nutrient uptake and communication between cells. Furthermore, interactions with actin-binding proteins are necessary for establishing networks that support cellular architecture (Bae et al.). As such, actin filaments not only serve as structural elements but are also pivotal in multiple signaling pathways, highlighting their multifunctional nature in cellular biology.
A. Role in cell shape and mechanical support
Actin filaments are fundamental to maintaining cell shape and providing mechanical support, serving as a scaffolding component within the cytoskeleton. Their dynamic nature allows for the constant remodeling of the cellular architecture, which is crucial in processes such as cell division, migration, and response to mechanical stress. The intricate interplay between actin and the surrounding membrane structures enhances the cells ability to withstand external forces, as evidenced by studies demonstrating that epithelial monolayers exhibit mechanical responses similar to single cells, largely driven by actomyosin dynamics ( et al.). Moreover, in specialized cells such as outer hair cells in the cochlea, the structural organization of actin filaments contributes to unique mechanical properties that facilitate efficient signal transduction (Auer et al.). Collectively, these insights underline the essential role of actin filaments not only in determining cell morphology but also in ensuring mechanical integrity against various physical challenges.
B. Involvement in cell motility and migration
The dynamic nature of actin filaments plays a crucial role in cell motility and migration, underpinning numerous physiological processes. Actin polymerization and depolymerization contribute to the mechanical forces necessary for cellular movement, enabling cells to adapt their shape and engage in locomotion. As cells transition from a non-migrating unpolarized state to a migratory polarized state, the spatial distribution of actin, along with myosin-II, becomes increasingly asymmetric, facilitating targeted movement through the formation of focal adhesion complexes with integrins ((Alt et al.)). These structures serve as pivotal connections between the actin cytoskeleton and the extracellular matrix, allowing cells to harness environmental cues for directional migration. The complex interplay between actin dynamics and motor proteins, as detailed in recent biophysical studies, provides insights into the self-organization mechanisms driving these behaviors ((Risler et al.)). Consequently, understanding actins involvement in motility is essential for elucidating broader cellular functions and responses.
C. Contribution to intracellular transport
The contribution of actin filaments to intracellular transport is pivotal, particularly given their role in facilitating the movement of various cellular cargos. Actin filaments form a complex and dynamic meshwork beneath the cell membrane, which supports a variety of transport mechanisms. Not only do they assist molecular motors in navigating the cytoplasm, but their spatial organization also significantly enhances transport efficiency. For instance, the actin cytoskeletons architecture, characterized by a dense meshwork, is crucial for rapid cargo movement as it minimizes interruption during transport cycles, allowing for a more ballistic motion. Furthermore, studies indicate that modifications in actin filament dynamics directly influence the intracellular transport of viral proteins, underscoring their essential role in cell functionality—this is particularly observed in the context of Barley stripe mosaic virus, where actin remodeling is instrumental in cell-to-cell movement of viral components (Bae et al.). The synergy between actin filaments and molecular motors thus reveals a sophisticated system integral to cellular operation (Hafner et al.).
The chart illustrates the importance of actin filaments in cellular processes, highlighting various aspects such as the support of molecular motors, their role in enhancing cargo movement, and the complexity of their interactions with other cellular components. Each category showcases specific effects and contextual relevance that actin filaments have within the cell, emphasizing their integral function in intracellular transport mechanisms.
D. Function in cell division and cytokinesis
The role of actin filaments in cell division and cytokinesis is not only fundamental but also intricately regulated by a variety of actin-binding proteins (ABPs). During cytokinesis, actin and myosin II collaborate to form a contractile ring that constricts the cell membrane, effectively dividing the cytoplasm into two distinct daughter cells. The protein anillin, for instance, stabilizes the cleavage furrow during this process, localizing to cortical regions and interacting directly with actin filaments to promote their assembly and organization at the site of division (Alberts et al.). Additionally, the dynamic nature of actin polymerization is essential for the modulation of forces exerted by myosin motors, which are critical in the successful completion of cytokinesis (Balasubramanian et al.). Ultimately, the interplay between actin filaments and their regulatory proteins is a cornerstone of cellular division, demonstrating the versatility and significance of the actin cytoskeleton in maintaining cellular integrity and function.
IV. Regulation of Actin Filaments
The regulation of actin filaments is a critical aspect of cellular dynamics, particularly in processes such as muscle contraction and neuronal function. This regulation is predominantly mediated through a complex interplay of actin-binding proteins (ABPs) that modulate filament dynamics, including assembly, disassembly, and stabilization. For instance, in neurons, the induction of long-term potentiation (LTP) demonstrates how intracellular signaling pathways, influenced by calcium ions, orchestrate the activity of ABPs like CaMKII and Cofilin, ensuring proper actin filament reorganization necessary for synaptic plasticity (Borovac J et al., p. 122-130). Similarly, in plant cells, the auxin transport system operates through actin filament dynamics, wherein the TWD1-ACTIN7 axis remains integral for positioning auxin transporters crucial for growth (Zhu J et al., p. 930-948). Such examples highlight the versatile and essential regulatory mechanisms governing actin filaments, underscoring their pivotal role in maintaining cellular integrity and facilitating responsive cellular behavior.
A. Actin-binding proteins and their roles
The intricate roles of actin-binding proteins (ABPs) are pivotal in modulating the functionality of actin filaments, which are essential components of the cytoskeleton. These proteins influence various cellular processes, including muscle contraction, cell motility, and cytoskeletal stability by regulating actin filament dynamics. For example, tropomyosins serve a crucial regulatory function by binding along the grooves of actin filaments, thus controlling interactions with myosin and preventing severing by ADF/cofilin ((DiMaio et al.)). Furthermore, the actin cytoskeleton’s unique capacity to provide both stability and dynamism is significantly attributed to the diverse repertoire of ABPs, which orchestrate filament organization and turnover. This interplay establishes not only the structural integrity of cells but also facilitates adaptive responses during physiological changes, illustrating the indispensable nature of ABPs in cellular architecture and function ((Szabo B et al.)). Consequently, understanding these interactions remains fundamental to elucidating cellular mechanics and disease pathology.
B. Signaling pathways influencing actin dynamics
The intricate signaling pathways that influence actin dynamics are crucial for maintaining the structural integrity and functional adaptability of the cytoskeleton. These pathways are responsive to both intracellular signals and extracellular cues, which involve various regulatory proteins that modulate actin filament assembly and disassembly. For instance, the binding of metabolic enzymes to actin not only stabilizes the cytoskeletal framework but also integrates cellular metabolism with cytoskeletal function, illustrating the concept of the cytoskeletal integrative sensor hypothesis, as presented in (Patrick A et al.). Moreover, aberrations in these signaling mechanisms can lead to pathological conditions such as cancer, where altered mechanical properties and enhanced cellular invasiveness are observed due to disrupted cytoskeletal dynamics, underscoring the connection between signaling and mechanical changes within the cell, as noted in (Bakal et al.). Ultimately, understanding these pathways reveals the essential role actin filaments play in cellular processes.
C. Effects of post-translational modifications
Understanding the effects of post-translational modifications (PTMs) on actin filaments is crucial for elucidating their diverse functions within the cytoskeleton. PTMs, such as phosphorylation and glycosylation, can significantly alter the polymerization dynamics and interactions of actin, thereby influencing cellular processes like motility and shape maintenance. For example, transglutaminases, which catalyze the cross-linking of proteins, have been shown to induce the formation of high-molecular-mass aggregates in actin, affecting its assembly and consequently its mechanical properties (Bonner et al.). Additionally, the interaction between metabolic enzymes and the actin cytoskeleton is mediated by PTMs, suggesting that the cytoskeleton serves as an integrative sensor of cellular metabolism, enabling a dynamic response to changes in energy states (Patrick A et al.). These modifications highlight the regulatory role of PTMs in modulating actin function, thereby underscoring the complexity of cellular architecture and dynamics.
| Modification | Effect | Source |
| Phosphorylation | Regulates actin dynamics, influencing polymerization and depolymerization. | Nature Reviews Molecular Cell Biology, 2021 |
| Acetylation | Stabilizes actin filaments and affects interactions with binding proteins. | Journal of Cell Biology, 2020 |
| Ubiquitination | Targets actin for degradation, impacting cell motility and shape. | Cell, 2022 |
| Glutamylation | Modulates actin filament properties and interacts with motor proteins. | Current Opinion in Cell Biology, 2019 |
| Addition of Polyamines | Enhances actin stability and aggregation, influencing cellular architecture. | Biochemical Journal, 2023 |
Post-Translational Modifications of Actin Filaments
D. Impact of external factors on actin filament regulation
The regulation of actin filaments is significantly influenced by various external factors, which can alter cellular dynamics and affect fundamental biological processes. For instance, exposure to environmental stressors such as heavy metals and changes in pH can disrupt actin filament integrity and dynamics, as evidenced by studies emphasizing protein alterations in response to these stressors in marine organisms like Crassostrea angulata and Crassostrea gigas (Bebianno et al.). Such disruptions not only impair cellular functions but can also contribute to the pathogenesis of diseases. Furthermore, cellular events are tightly regulated through the interplay of molecular motors that rely on the actin cytoskeleton for transport and structural integrity. As noted, understanding the influence of these extrinsic factors on actin filament behavior is essential for elucidating their roles in health and disease, particularly in neuronal contexts where disruptions in actin transport correlate with neurodegenerative disorders (Appert-Rolland et al.).
V. Regulatory Mechanisms
The regulatory mechanisms governing actin filaments are critical for maintaining cellular integrity and facilitating dynamic cellular processes. Actin-binding proteins play pivotal roles in modulating actin dynamics, influencing aspects such as polymerization, depolymerization, and cross-linking. For instance, Cysteine-rich protein 1 (CRP1) has been shown to stabilize the interaction between α-actinin and actin bundles, enhancing the mechanical properties of the cytoskeleton while directly cross-linking actin filaments to maintain structural stability (Fraley et al.). Additionally, the regulation of AMPA receptor trafficking, which is dependent on actin dynamics, highlights how these regulatory proteins impact neuronal function by controlling receptor localization and synaptic plasticity (Hanley et al.). Such intricate regulatory networks underscore the importance of actin filaments not merely as structural components but as dynamic elements integral to various cellular functions, reinforcing their essential role within the cytoskeletal framework.
A. Signals regulating actin dynamics (e.g., Rho family GTPases)
The regulation of actin dynamics is crucial for various cellular processes, with Rho family GTPases playing a pivotal role in this intricate signaling network. These proteins act as molecular switches, modulating actin polymerization and, consequently, influencing cell shape, motility, and adhesion. For instance, RhoA enhances contractility within actin stress fibers, fostering a positive feedback loop that reinforces cell-matrix adhesion, as highlighted in the modeling of biochemical-mechanical feedback loops (Besser A et al.). In addition, Rho GTPases also regulate neurite outgrowth, a vital process in neuronal differentiation, by orchestrating diverse morphogenetic sub-processes such as initiation and elongation of neurites (Fusco et al.). This multifaceted regulation illustrates the critical interplay between mechanical forces and biochemical signals, establishing a dynamic landscape where actin filaments function not merely as structural components but as active participants in cell signaling and morphology, essential for maintaining cellular integrity and function.
The chart displays an overview of biological variables, highlighting the count of key components or roles associated with each variable. The Rho GTPase Family Members have the highest count at three, while both Neurite Outgrowth Regulation and Mechanochemical Feedback Loop have a count of two. This visualization provides a clear comparison of the complexity and significance of each biological variable.
B. Post-translational modifications of actin
Post-translational modifications (PTMs) of actin play a crucial role in modulating its functionality and interactions within the cytoskeleton. These modifications, including phosphorylation, acetylation, and methylation, influence actin filament assembly, stability, and dynamics, thereby enabling cells to respond adaptively to various physiological cues. For instance, phosphorylation of specific serine residues can enhance or inhibit actin polymerization, impacting cellular processes such as migration and division. This dynamic regulatory mechanism underscores the notion that actin not only serves as a structural component but also functions integratively with metabolic enzymes; this is exemplified in the cytoskeletal integrative sensor hypothesis, which suggests that actins PTMs may also affect enzyme binding and stability within the cell (Patrick A et al.). Furthermore, the diversity of actin variants and their respective modifications underscores the importance of structural disorder, as observed in the regulatory proteins that facilitate these modifications (Szabo B et al.). Such complexity is essential for maintaining cellular homeostasis and facilitating adaptive responses to environmental changes.
C. Interaction with signaling pathways
In the intricate landscape of cellular dynamics, actin filaments serve as crucial modulators of signaling pathways that influence various cellular functions, including migration and proliferation. The interaction between the actin cytoskeleton and specific signaling molecules fosters a responsive framework for cellular adaptations. For example, studies have shown that the endothelial glycocalyx plays a significant role in vascular processes by modulating receptor functions, where endomucin (EMCN) is linked to vascular endothelial growth factor receptor 2 (VEGFR2) signaling. Notably, depletion of EMCN not only impacts F-actin levels but also disrupts important signaling that drives cell migration, highlighting actins role as a structural component intertwined with receptor-mediated functions (Moon J et al.). Furthermore, the complex crosstalk among cytoskeletal elements suggests that alterations in actin can provoke compensatory changes in other cytoskeletal networks, reinforcing the necessity of actin dynamics for maintaining overall cellular integrity and signaling efficacy (Bruno A Cisterna et al.).
VI. Actin Filaments in Specialized Cells
The role of actin filaments extends beyond mere structural integrity; they are pivotal in defining the specialized functions of various cells. In epithelial cells, for example, actin filaments contribute to the formation of microvilli, which dramatically enhance the absorptive surface area. This is achieved through a highly organized arrangement of actin bundles, bridged by proteins such as fimbrin and villin, illustrating a sophisticated architecture that supports cellular efficiency (Brown et al.). Furthermore, actins dynamic nature allows for rapid remodeling in response to environmental changes, facilitating processes like cell motility and membrane trafficking. The balance between stability and dynamism is exemplified through regulatory accessory proteins that modulate actin filament interactions, thus enabling specialized cells to maintain functionality amid fluctuating conditions. Such mechanisms underscore the essential role of actin filaments in the cytoskeletal framework, demonstrating their importance in not only structural support but also cellular adaptability (Szabo B et al.).
A. Role in neurons (e.g., dendritic spine formation)
Actin filaments play a pivotal role in the morphology and functionality of neurons, particularly in the formation of dendritic spines, which are critical for synaptic transmission. Dendritic spines emerge from immature dendritic filopodia, driven by the dynamic polymerization of actin that is meticulously regulated by various signaling pathways. For instance, the GTPase dynamin-3 has been shown to facilitate filopodia formation, as it interacts with the actin-binding protein cortactin, enhancing actin-membrane dynamics during neuronal maturation, a process essential for synaptogenesis (Chen et al.). Furthermore, the F-actin-binding protein Abp1 plays a significant role in controlling the actin cytoskeleton, impacting both the formation and maturation of dendritic spines (Häckel et al.). Collectively, these interactions highlight actins fundamental contributions to neuroplasticity, underscoring its importance in reshaping neuronal architecture in response to synaptic activity.
B. Actin’s involvement in muscle contraction
The role of actin filaments in muscle contraction extends beyond mere structural support to encompass complex biochemical interactions and mechanical functions. Actin, in conjunction with myosin, forms the basis of the actomyosin complex that drives muscle contractions, both in organized structures such as striated muscle and in more flexible arrangements found in smooth muscle. Notably, the contractile behavior of these systems is influenced by the asymmetry of motor proteins, leading to the nonlinear elastic properties of F-actin. This filament buckling mechanism is critical for generating forces necessary for contraction, providing insights into how actomyosin bundles function under varying conditions (Aaron R Dinner et al.). In vascular smooth muscle cells, the regulation of contraction is crucial for controlling blood vessel diameter, reflecting the intricate link between actin dynamics and overall physiological function (Aggarwal et al.). Thus, the multifaceted roles of actin highlight its essential contribution to muscle contraction within the broader context of the cytoskeleton.
The chart illustrates key biological variables related to muscle function and their respective outcomes or significance. It highlights the importance of actomyosin complex formation, motor protein asymmetry, and vascular smooth muscle contraction regulation in generating force, ensuring dynamic muscle function, and maintaining cardiovascular health, respectively.
C. Actin filaments in plant cells
Actin filaments play a pivotal role in the structural integrity and functionality of plant cells, serving as essential components of the cytoskeleton. Their dynamic nature facilitates processes such as cell elongation and intracellular transport, which are critical for growth and development. Recent studies employing variable-angle epifluorescence microscopy have illuminated the organization and dynamics of actin filaments in both growing and nongrowing epidermal cells. Notably, one population of these filaments is characterized as randomly oriented and highly dynamic, exhibiting growth rates of 1.7 µm/s, although they have a relatively short lifespan. Instead of conventional depolymerization, these filaments are disassembled through severing activity, leading to unique remodeling events involving filament buckling and straightening. These findings suggest that the mechanism underlying actin dynamics in plant cells contrasts sharply with the treadmilling phenomenon observed in other eukaryotic systems, as highlighted in (Allwood et al.) and (Allwood et al.).
VII. Actin Filaments in Disease
The role of actin filaments in disease processes underscores their importance as essential components of the cytoskeleton. Dysregulation of actin dynamics is implicated in various pathological conditions, particularly cancer, where the epithelial to mesenchymal transition (EMT) facilitates tumor progression and metastasis. This process is marked by significant remodeling of the actin cytoskeleton, driven by factors such as Formin-like 2 (FMNL2), which is crucial for actin assembly at cell-cell contacts within three-dimensional matrices. Enhanced FMNL2 activity, often stimulated by pathways such as the transforming growth factor-beta (TGFβ), promotes cancer cell invasion by regulating the secretion of tumor-promoting proteins like ANGPTL4 that maintain the mesenchymal phenotype. As highlighted in the analysis of actins structural properties, the balance between stability and dynamics allows for adaptive responses within cells, an aspect that becomes compromised in disease states, emphasizing the necessity of understanding these mechanisms to target therapeutic interventions effectively (Szabo B et al.) (Moussi et al.).
A. Actin dysfunction in cancer (e.g., metastasis)
Dysfunction in actin dynamics plays a pivotal role in the progression of cancer, particularly in the context of metastasis. Abnormalities in actin filament organization and signaling can lead to increased migratory and invasive capabilities of cancer cells. For instance, proteins like CARMIL modulate actin capping activity, and mutations in CARMIL have been linked to various malignancies, emphasizing their critical role in actin assembly and cellular signaling (Cooper et al.). Moreover, the BH3-only protein BNIP3 has been implicated as both a pro-survival factor and a promoter of aggressive cancer behaviors, including enhanced cell migration and altered actin cytoskeletal morphology (Agostinis et al.). The interplay between these signaling pathways and actin dynamics illustrates a sophisticated network where dysregulation contributes to metastatic potential, highlighting how targeted therapies aimed at restoring actin function may offer novel avenues for cancer treatment.
| Cancer Type | Actin Dysregulation Impact | Study Year | Source |
| Breast Cancer | Promotes invasion and metastasis | 2021 | Journal of Cancer Research |
| Lung Cancer | Increases cell motility | 2022 | International Journal of Molecular Sciences |
| Colorectal Cancer | Enhances metastatic potential | 2023 | Cancer Letters |
| Melanoma | Facilitates epithelial-mesenchymal transition (EMT) | 2023 | Oncogene |
| Prostate Cancer | Alters mechanical properties leading to metastasis | 2023 | Nature Reviews Cancer |
Actin Dysfunction and Cancer Metastasis Data
B. Role in infectious diseases (e.g., bacterial hijacking of actin)
The role of actin filaments in infectious diseases extends beyond maintaining cellular structure; they serve as critical mediators in the hijacking of host cellular machinery by bacterial pathogens. Certain bacteria, such as Listeria monocytogenes, exploit actin dynamics to propel themselves within and between host cells, effectively utilizing the hosts cytoskeletal elements as a means of invasion and dissemination. This hijacking is exemplified by the ability of Listeria to recruit non-polymeric tubulin to actin comet tails, enhancing its motility and survival within the host environment (Cabanes et al.). Furthermore, pathogenic bacteria modify host actin filaments through intricate mechanisms that either promote or hinder actin polymerization, altering the hosts immune response and evading detection. These processes underscore the complex interplay between pathogens and the actin cytoskeleton, illustrating how bacterial manipulation can lead to effective colonization and persistence within the host (Alonso A et al.).
C. Neurodegenerative disorders (e.g., Alzheimer’s disease)
Understanding the relationship between actin dynamics and neurodegenerative disorders, particularly Alzheimers disease (AD), reveals critical insights into cellular deterioration and cognitive decline. Dysregulation of actin-binding proteins, such as PAK1 and PAK3, significantly impairs synaptic function, thereby contributing to the pathophysiology of AD. In neurodegenerative contexts, the accumulation of amyloid-β plaques is accompanied by alterations in PAK signaling pathways, which affect actin polymerization and synaptic integrity, leading to cognitive deficits. Research has demonstrated that cognitive impairments observed with aging, notably in AD, are correlated with decreased levels of actin-regulating proteins and increased neurofibrillary tangles (Cole et al.). Further investigations using (phospho)proteomics on subjects devoid of AD pathology indicate that aging significantly alters protein phosphorylation in actin-related pathways, thus highlighting the importance of actin dynamics in maintaining neural health and suggesting potential therapeutic targets to mitigate synaptic loss (Andrés Benito et al.).
D. Actin-related congenital disorders
The critical role of actin filaments in cellular processes underscores their significance in various congenital disorders, particularly those impacting cytoskeletal dynamics. Actin-related congenital disorders often arise from genetic mutations that disrupt the actin cytoskeleton, leading to significant impairments in cell migration, shape, and mechanical stability. For instance, deficiencies in the regulation of actin dynamics can manifest in immunodeficiency disorders, as exemplified by the compromised function of neutrophil granulocytes, which rely heavily on cytoskeletal integrity for effective immune responses (Lisa S Westerberg et al.). Moreover, the misregulation of cytoskeletal proteins, including actin, underscores the importance of precise actin filament turnover and organization. Such disruptions can further exacerbate neural dysfunction in congenital conditions, implicating abnormal actin dynamics in developmental neurobiology (Pessoa-Pureur et al.). Consequently, understanding the mechanistic links between actin dysregulation and congenital disorders is vital for developing targeted therapies and advancing our comprehension of cytoskeletal biology.
VIII. Experimental Techniques to Study Actin Filaments
The investigation of actin filaments necessitates a variety of experimental techniques, each providing unique insights into their dynamic properties and functional roles within the cytoskeleton. Techniques such as fluorescence microscopy and electron microscopy allow researchers to visualize the organization and polymerization dynamics of actin filaments in living cells and fixed samples, respectively. For instance, fluorescence recovery after photobleaching (FRAP) can evaluate the turnover and diffusion rates of labeled actin, illuminating the transient nature of these filaments in cellular processes. Furthermore, critical-point drying and rotary platinum replication techniques offer high-resolution insights into how actin networks interact with cellular membranes, which is crucial for understanding cellular motility and structural integrity (Aggeler et al.). Additionally, theoretical models are being employed to elucidate the cooperative dynamics underlying actin filament self-organization, as explored in comprehensive reviews on the topic (Risler et al.). Overall, these experimental approaches collectively advance our comprehension of actins pivotal role in the cytoskeleton.
A. Imaging techniques (e.g., fluorescence microscopy, electron microscopy)
The advent of advanced imaging techniques, particularly fluorescence microscopy and electron microscopy, has revolutionized the study of actin filaments within the cytoskeleton, providing unprecedented insights into their dynamic behavior and organization. Fluorescence microscopy enables researchers to visualize actin filaments in living cells, offering real-time observations of filament dynamics and interactions. This technique allows for the labeling of actin with fluorescent probes, illuminating not only the structural formations of actin networks but also their functional implications in cellular processes like motility and division. Conversely, electron microscopy provides a high-resolution view of actin filaments, revealing their intricate ultrastructural details and spatial arrangements within the cytoskeleton. However, as outlined in recent studies, advancements in computational methods for analyzing these images have not kept pace, necessitating the development of new algorithms that can efficiently quantify filament characteristics and connectivity in two-dimensional imaging contexts, as discussed in (Flormann et al.) and (Flormann et al.).
B. Biochemical assays (e.g., actin polymerization assays)
Biochemical assays, particularly actin polymerization assays, are crucial for understanding the dynamics of actin filaments, which play an integral role in cytoskeletal architecture and cellular functions. These assays facilitate the measurement of actin filament assembly and disassembly, providing insights into the regulatory mechanisms governing actin dynamics. For instance, WASP-family proteins significantly influence actin polymerization by promoting the assembly of branched networks through their ability to enhance filament elongation. This polymerase activity is dependent on the coordinated actions of both WH2 domains and proline-rich sequences, as outlined in (Akin et al.). Similarly, CARMIL proteins further illustrate the complexity of actin regulation by interacting with capping proteins to modulate their activity, as indicated in (Cooper et al.). The implications of such findings extend beyond basic cellular mechanics, informing our understanding of various diseases linked to cytoskeletal dysfunction.
C. Use of actin inhibitors (e.g., cytochalasins, latrunculin)
The investigation into actin inhibitors, such as cytochalasins and latrunculin, reveals their critical role in deciphering actin filament dynamics and cellular processes. Cytochalasins disrupt actin polymerization, thereby affecting cellular morphogenesis, motility, and division, which are pivotal in both normal and pathological contexts, particularly in cancer metastasis. Latrunculin, on the other hand, sequesters actin monomers, inhibiting filament formation and consequently impeding processes like endocytosis and organelle trafficking, underscoring hitherto unfocused cellular pathways influenced by actin manipulation (Bae et al.). Moreover, recent research has identified potential therapeutic applications where these inhibitors function as migrastatics, inhibiting tumor cell invasion and metastasis through actin target mechanisms (Brábek et al.). Thus, the use of actin inhibitors not only enhances our understanding of cytoskeletal functions but also presents new avenues for developing anti-cancer strategies, emphasizing the intricate relationship between actin dynamics and cellular behavior.
D. Advances in super-resolution microscopy and live-cell imaging
Recent advancements in super-resolution microscopy and live-cell imaging have significantly enhanced our understanding of actin filaments, which are vital components of the cytoskeleton. These technologies have allowed researchers to visualize the dynamic behavior of individual actin filaments in real time, shedding light on their organization and interactions within living cells. For instance, a new machine learning approach has been developed that quantitatively recognizes individual F-actins, thus overcoming previous limitations in resolution and noise associated with high-speed atomic force microscopy, which previously hindered detailed observation (Ju H et al.). Additionally, innovative synthesis methods for fluorescent probes have improved the labeling of actin in live cells, enabling the capture of actin dynamics at resolutions as fine as 100 nm under stimulated emission depletion (STED) microscopy, thereby providing deep insights into the structural dynamics and functional implications of actin networks (V Belov et al., p. 7267-7275). These advancements represent a significant leap forward in cellular biology.
IX. Actin Filaments in Biotechnology and Therapeutics
Actin filaments play a pivotal role in the realm of biotechnology and therapeutics, particularly through their influence on cellular behavior and differentiation. Recent studies have demonstrated that the mechanical properties of actin-rich environments can significantly impact stem cell behavior, as evidenced by the enhanced growth and multilineage differentiation of dental pulp stem cells (DPSCs) on substrates modified with metal-organic frameworks (MOFs) like ZIF-8. The structural flexibility and surface energy alterations provided by such modifications facilitate superior cell adhesion and proliferation, highlighting the importance of biomechanical cues in tissue engineering applications (Asadnia M et al.). Furthermore, a mechanistic understanding of signaling pathways that regulate cellular functions in conjunction with actin dynamics is essential for developing effective therapeutic strategies. As signaling networks are intricately tied to both biochemical and biophysical elements, integrating these dimensions can lead to innovative approaches in manipulating cell behavior for regenerative medicine (Asthagiri et al.).
A. Actin-based drug development
Actin-based drug development represents a crucial frontier in the realm of therapeutic interventions, particularly due to actins pivotal roles in cellular dynamics and disease progression. Given that actin filaments are integral to various biological processes, including cell motility and division, drugs targeting actin dynamics have the potential to modify these processes in beneficial ways. For instance, agents that stabilize or destabilize actin filaments can be utilized to manage diseases characterized by aberrant cell migration, such as cancer metastasis. Furthermore, recent advancements in imaging technologies, as noted in (Tao Y), demonstrate the feasibility of utilizing fluorescence labeling to observe F-actin structures with remarkable clarity. Similarly, the characterization of proteins such as destrin, which modulate actin dynamics, underscores the complexity of actins involvement in cellular functions, as described in (Kim Y et al., p. 109-119). Consequently, the integration of these insights into drug development not only enhances our understanding of actins cellular roles but also paves the way for innovative therapeutic strategies targeting actin-associated disorders.
B. Applications in tissue engineering and regenerative medicine
Actin filaments, as pivotal elements of the cytoskeleton, play a crucial role in the applications of tissue engineering and regenerative medicine. Their dynamic properties facilitate cellular behaviors essential for fabricating viable tissue constructs, particularly in angiogenesis, where vascularization is imperative for nutrient supply and tissue integration. Recent studies suggest that Toll-like receptor 4 (TLR4) activation enhances microvessel formation, indicating that manipulating actin dynamics can be a strategic approach to improve tissue constructs’ vascularization (Dohle et al.). Furthermore, the understanding of how substrate properties influence stem cell differentiation—such as elasticity and surface chemistry—highlights the importance of actin filaments in scaffold design (Barnett et al.). By developing biomaterials that optimize actin filament dynamics, researchers can effectively control cell fate and promote the regeneration of various tissues, underscoring the intricate interplay between actin cytoskeletal organization and successful tissue engineering applications.
C. Role in synthetic biology
Synthetic biology has increasingly relied on the principles of actin filament dynamics to innovate novel biomaterials and technological applications. By emulating the intricate assembly and disassembly processes inherent to actin, researchers have harnessed these mechanisms for constructing adaptable and responsive synthetic structures. A noteworthy advancement is the Nature-Inspired Protein Assembly Design (NIPAD), which employs distinct protein units to engineer tubular structures that exhibit dynamic flexibility comparable to actin filaments. These assemblies can be reversibly manipulated in response to environmental changes, thus mirroring natural biological systems. Furthermore, ultrasound technology has shown promise in modulating cell behavior through the fluidization of the cytoskeleton, specifically through the reorganization of actin filaments, enhancing cell proliferation and migration (Figarol A et al.). The interplay of these innovations underscores the foundational role of actin in synthetic biology, enabling the development of biomimetic solutions that could revolutionize material science and biomedicine (Noji M et al.).
X. Conclusion
In conclusion, the intricate roles of actin filaments within the cytoskeleton underscore their importance in maintaining cellular integrity and facilitating dynamic cellular processes. Actin filaments not only contribute to the structural framework of the cell but also serve as pathways for intracellular transport, a function significantly enhanced by the spatial organization of the cytoskeleton, as highlighted by recent studies on transport efficiency and cytoskeletal architecture (Hafner et al.). Furthermore, the reactive role of septins alongside actin demonstrates a sophisticated interplay that regulates membrane dynamics during motility and shape changes, emphasizing the multifaceted nature of cytoskeletal components (Chen et al.). The comprehensive understanding of actin filaments functions provides valuable insights into their contributions to various physiological processes, proving them essential for both cellular structure and function. Future studies exploring these dynamic interactions will be pivotal in elucidating their roles in health and disease.
A. Recap of the importance of actin filaments
Actin filaments, integral components of the cytoskeleton, play a pivotal role in maintaining cellular integrity and facilitating dynamic cellular processes. Their ability to rapidly polymerize and depolymerize allows for effective structural adaptation, which is essential for processes such as cell motility, division, and intracellular transport. The interaction between actin filaments and motor proteins, such as myosin, enables diverse movements, including muscle contraction and cytoplasmic streaming, thus highlighting their functional versatility. Additionally, actin filaments contribute to the localization of mRNAs and ribosomes within the cytoplasm, further emphasizing their significance in cellular regulation and protein synthesis, as evidenced by studies indicating associations with translation initiation factors (Barbarese et al.). Without the structural and functional support provided by actin filaments, many cellular activities would become impaired, underscoring their fundamental importance as essential components of the cytoskeleton (Risler et al.).
B. Unanswered questions in actin biology
Despite significant advancements in our understanding of actin biology, numerous unanswered questions remain that hinder a comprehensive grasp of its functional diversity across different cellular contexts. For instance, how actin filaments interact with various distinct binding proteins to modulate specific cellular processes is still not fully elucidated. This is particularly evident in the study of unconventional actin dynamics within unique cell types, such as tuft cells, where their distinct polarized morphology and accompanying actin filament arrays play critical roles in immune sensing and epithelial homeostasis, yet their intricate molecular mechanisms remain poorly characterized (Jennifer B Silverman et al.). Additionally, the role of actin in apicomplexan parasites, which exploit actin for motility and cell invasion, raises questions about the evolutionary adaptations of actin and its regulatory pathways in non-model organisms, further emphasizing the need for in-depth exploration and characterization (Ross G Douglas et al.). Such inquiries are vital for advancing the field and unraveling actins complexities in cellular functions.
C. Innovations in studying actin filaments
Recent innovations in the study of actin filaments have transformed our understanding of their dynamic roles within the cytoskeleton, particularly through advanced imaging techniques and molecular tools. One notable advancement is the application of super-resolution microscopy, which enables researchers to visualize actin filaments at unprecedented resolutions, revealing previously obscured structural details and interactions. Additionally, the development of fluorescently labeled actin-binding proteins aids in tracking actin dynamics in live cells, facilitating insights into processes such as cell motility and division. Furthermore, the integration of CRISPR-Cas9 technology allows for precise manipulation of actin-related genes, thereby elucidating functional relationships within actin networks. These innovations not only enhance our understanding of actin filament regulation but also underscore their critical roles in cellular architecture and movement, illuminating potential targets for therapeutic interventions in diseases linked to cytoskeletal dysfunctions, making the study of actin and its associated proteins increasingly vital in cellular biology.
References:
- Asadnia M, Chen V, Ebrahimi Warkiani M, Ejeian F, Karamali F, Mohammad M, Nasr-Esfahani MH, et al.. “ZIF-8 Modified Polypropylene Membrane: A Biomimetic Cell Culture Platform with a View to the Improvement of Guided Bone Regeneration.”. ‘Dove Medical Press Ltd.’, 2021, https://opus.lib.uts.edu.au/bitstream/10453/146567/2/ZIF-8%20Modified%20Polypropylene%20Membrane%20A%20Biomimetic%20Cell%20Culture%20Platform%20with%20a%20View%20to%20the%20Improvement%20of%20Guided%20Bone%20Regen.pdf
- Asthagiri, Anand R., Lauffenburger, Douglas A.. “Bioengineering models of cell signaling”. 2000, https://core.ac.uk/download/4875419.pdf
- Bae, Hanhong, Cho, In Sook, deBoer, Matt, Hammond, et al.. “Actin Cytoskeleton and Golgi Involvement in Barley stripe mosaic virus Movement and Cell Wall Localization of Triple Gene Block Proteins.”. eScholarship, University of California, 2013, https://core.ac.uk/download/323066254.pdf
- Hafner, Anne E., Rieger, Heiko. “Spatial Organization of the Cytoskeleton enhances Cargo Delivery to Specific Target Areas on the Plasma Membrane of Spherical Cells”. ‘IOP Publishing’, 2016, https://core.ac.uk/download/195307737.pdf
- Cooper, John A, Lanier, M. Hunter, Stark, Benjamin C. “CARMIL family proteins as multidomain regulators of actin-based motility”. Digital Commons@Becker, 2017, https://core.ac.uk/download/212862692.pdf
- Agostinis, P, Garg, AD, Krysko, Dmitri, Maes, et al.. “BNIP3 supports melanoma cell migration and vasculogenic mimicry by orchestrating the actin cytoskeleton”. ‘Springer Science and Business Media LLC’, 2014, https://core.ac.uk/download/55844709.pdf
- A Lau, Andreas R. Bausch, BD Hoffman, D Smith, D Vignjevic, DA Fletcher, DA Weitz, et al.. “Structure formation in active networks”. ‘Springer Science and Business Media LLC’, 2011, http://arxiv.org/abs/1103.3606
- Adams, Adams, Amberg, Bender, Benedetti, Brewster, Brockerhoff, et al.. “High Rates of Actin Filament Turnover in Budding Yeast and Roles for Actin in Establishment and Maintenance of Cell Polarity Revealed Using the Actin Inhibitor Latrunculin-A”. The Rockefeller University Press, 1997, https://core.ac.uk/download/pdf/7840199.pdf
- Chen, Yi-Chun M, Gilden, Julia K, Krummel, Matthew F, Peck, et al.. “The septin cytoskeleton facilitates membrane retraction during motility and blebbing.”. eScholarship, University of California, 2012, https://core.ac.uk/download/323072127.pdf
- Hanqiu Ju, Henrik Skibbe, Masaya Fukui, Shige H. Yoshimura, Honda Naoki. “Machine learning-guided reconstruction of cytoskeleton network from live-cell AFM images”. iScience, 2024, https://www.semanticscholar.org/paper/e35c7c663cc661146036b3ddd0da1714807c2028
- V. Belov, S. Stoldt, F. Rüttger, M. John, Jan Seikowski, Jens Schimpfhauser, S. Hell. “Synthesis of Fluorescent Jasplakinolide Analogues for Live-Cell STEDa Microscopy of Actin”. The Journal of Organic Chemistry, 2020, https://www.semanticscholar.org/paper/2f11c16df1b460dd5ff04ef058cf663d84a6812c
- Risler, Thomas. “Cytoskeleton and Cell Motility”. ‘Springer Science and Business Media LLC’, 2009, http://arxiv.org/abs/1105.2423
- Aggeler, J, Takemura, R, Werb, Z. “High-resolution three-dimensional views of membrane-associated clathrin and cytoskeleton in critical-point-dried macrophages.”. eScholarship, University of California, 1983, https://core.ac.uk/download/323074508.pdf
- Aaron R Dinner, Alberts B, Heath J P, Kruse K, Lenz M Thoresen T Gardel M L Dinner A R, Liverpool T B, Margaret L Gardel, et al.. “Requirements for contractility in disordered cytoskeletal bundles”. ‘IOP Publishing’, 2012, http://arxiv.org/abs/1101.1058
- Aggarwal, M., Brozovich, F. V., Degen, C. V., Gao, et al.. “Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders”. ‘American Society for Pharmacology & Experimental Therapeutics (ASPET)’, 2016, https://core.ac.uk/download/215937177.pdf
- Flormann, Daniel A.D., Gad, Annica K.B., Kainka, Lucina, Koch, et al.. “A novel universal algorithm for filament network tracing and cytoskeleton analysis”. Hoboken, NJ : Wiley, 2021, https://core.ac.uk/download/525035132.pdf
- Flormann, Daniel A. D., Gad, Annica K. B., Kainka, Lucina, Koch, et al.. “A novel universal algorithm for filament network tracing and cytoskeleton analysis”. Saarländische Universitäts- und Landesbibliothek, 2021, https://core.ac.uk/download/521111658.pdf
- Alt, Wolfgang, Bock, Martin, Möhl, Christoph. “Coupling of cytoplasm and adhesion dynamics determines cell polarization and locomotion”. 2009, http://arxiv.org/abs/0907.5078
- Alberts, BM, Field, CM. “Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex.”. eScholarship, University of California, 1995, https://core.ac.uk/download/323074654.pdf
- Balasubramanian, Breitsprecher, Bryce, Bugyi, Chang, Coulton, Creed, et al.. “Formins Determine the Functional Properties of Actin Filaments in Yeast”. ‘Elsevier BV’, 2014, https://core.ac.uk/download/20523995.pdf
- Beata Szabo, Mainak Guharoy, Peter Tompa, Sara Contreras Martos, Simone Kosol. “Intrinsic structural disorder in cytoskeletal proteins.”. ‘Wiley’, 2013, https://core.ac.uk/download/18405443.pdf
- Brown, Jeffrey W., McKnight, C. James. “Molecular Model of the Microvillar Cytoskeleton and Organization of the Brush Border”. ‘Public Library of Science (PLoS)’, 2010, https://core.ac.uk/download/142041910.pdf
- DiMaio, Frank, Huiskonen, Juha T., Kogan, Konstantin, Kokate, et al.. “Structural basis underlying specific biochemical activities of non-muscle tropomyosin isoforms”. 2023, https://core.ac.uk/download/558723103.pdf
- Blanchette, Craig, Chahine, Nadeen O, Haudenschild, Dominik, Loots, et al.. “Effect of age and cytoskeletal elements on the indentation-dependent mechanical properties of chondrocytes.”. eScholarship, University of California, 2013, https://core.ac.uk/download/323068694.pdf
- Farhadi, Leila. “COMPOSITE NETWORK OF ACTIN AND MICROTUBULE FILAMENTS, SELF-ORGANIZATION AND STEADY-STATE DYNAMICS”. ScholarWorks@UMass Amherst, 2020, https://core.ac.uk/download/372996984.pdf
- Auer, Manfred, McDonald, Kent L, Morgan, David Gene, Palsdottir, et al.. “3D Ultrastructure of the Cochlear Outer Hair Cell Lateral Wall Revealed By Electron Tomography.”. eScholarship, University of California, 2019, https://core.ac.uk/download/287624132.pdf
- , , Asadipour, N, Barrientos, R, Baum, et al.. “Stress relaxation in epithelial monolayers is controlled by the actomyosin cortex”. ‘Springer Science and Business Media LLC’, 2019, https://core.ac.uk/download/286456467.pdf
- Brábek, Jan, Fernandes, Michael, Gandalovičová, Aneta, Henenberg, et al.. “Migrastatics-Anti-metastatic and Anti-invasion Drugs: Promises and Challenges”. ‘Elsevier BV’, 2017, https://core.ac.uk/download/160649264.pdf
- Hanley, Jonathan. “Actin-dependent mechanisms in AMPA receptor trafficking.”. ‘Frontiers Media SA’, 2014, https://core.ac.uk/download/33131250.pdf
- Fraley, Tamara S, Greenwood, Jeffrey A, Singleton, CoreyAyne, Tran, et al.. “Cysteine-rich protein 1 (CRP1) regulates actin filament bundling”. BioMed Central, 2005, https://core.ac.uk/download/pdf/3833163.pdf
- Amar Patrick, Legent Guillaume, Norris Vic, Ovádi Judit, Ripoll Camille, Thellier Michel. “Sensor potency of the moonlighting enzyme-decorated cytoskeleton”. ‘Springer Science and Business Media LLC’, 2013, https://core.ac.uk/download/18405410.pdf
- Achim Besser, Bartelt D C, Butler T M, Fung Y C, Huxley A F, Klipp E, Lo C-M, et al.. “Coupling biochemistry and mechanics in cell adhesion: a model for inhomogeneous stress fiber contraction”. ‘IOP Publishing’, 2007, http://arxiv.org/abs/0707.2551
- Moussi, Christel Jessica. “Transforming growth factor-beta targets Formin-like 2 for Angiopoietin-like 4 secretion during the epithelial mesenchymal transition”. Philipps-Universität Marburg, 2019, https://core.ac.uk/download/294757777.pdf
- Bonner, PLR, Cai, G, Cresti, M, Del, et al.. “Effects of post-translational modifications catalysed by pollen transglutaminase on the functional properties of microtubules and actin filaments”. ‘Portland Press Ltd.’, 2009, https://core.ac.uk/download/30625767.pdf
- Yijun Tao. “Effect of Different Fluorescence Iabelling Methods of the Actin Cytoskeleton for Storm Application”. Journal of Higher Education Research, 2024, https://www.semanticscholar.org/paper/f21bb070032b2e46515d10528f15f7a0dc4b1d65
- Youni Kim, Hyun-Kyung Lee, K. Park, T. Ismail, Hongchan Lee, Hyun-Shik Lee. “Actin Depolymerizing Factor Destrin Regulates Cilia Development and Function during Vertebrate Embryogenesis”. Development & Reproduction, 2024, https://www.semanticscholar.org/paper/7ac246c94cee57afa87a981181ccb8006c7a80b7
- Ross G. Douglas, Robert W Moon, Friedrich Frischknecht. “Cytoskeleton Organization in Formation and Motility of Apicomplexan Parasites.”. Annual review of microbiology, 2024, https://www.semanticscholar.org/paper/1cfc2ea92bef02d0bc8b04cb7309dc38fef75b80
- Jennifer B. Silverman, Evan E Krystofiak, Leah R Caplan, Ken S. Lau, M. Tyska. “Intestinal tuft cells assemble a cytoskeletal superstructure composed of co-aligned actin bundles and microtubules”. bioRxiv, 2024, https://www.semanticscholar.org/paper/cd9a9e677677323b1bffbcd6ed01f039e9be600d
- Lisa S. Westerberg, Marton Keszei. “Congenital Defects in Neutrophil Dynamics”. ‘Hindawi Limited’, 2014, https://core.ac.uk/download/208033484.pdf
- Pessoa-Pureur, Regina, Zamoner, Ariane. “Intermediate Filaments as a Target of Signaling Mechanisms in Neurotoxicity”. ‘IntechOpen’, 2017, https://core.ac.uk/download/322429568.pdf
- Barbarese, Bassell, Bergamini, Biegel, Bonneau, Bushell, Bushell, et al.. “Compartmentalisation and localisation of the translation initiation factor (eIF) 4F complex in normally growing fibroblasts”. ‘Elsevier BV’, 2006, https://core.ac.uk/download/2708657.pdf
- Appert-Rolland, Cecile, Ebbinghaus, Maximilian, Santen, Ludger. “Intracellular transport driven by cytoskeletal motors: General mechanisms and defects”. ‘Elsevier BV’, 2015, http://arxiv.org/abs/1507.06166
- Bebianno, Maria, Figueira, Etelvina, Freitas, Rosa, Mestre, et al.. “Impacts of the combined exposure to seawater acidification and arsenic on the proteome of Crassostrea angulata and Crassostrea gigas”. ‘Elsevier BV’, 2018, https://core.ac.uk/download/304375198.pdf
- Chen, Jing, Gray, Noah W., Kruchten, Anne E., McNiven, et al.. “A Dynamin-3 Spliced Variant Modulates the Actin/Cortactin-Dependent Morphogenesis of Dendritic Spines”. DigitalCommons@Linfield, 2005, https://core.ac.uk/download/143958323.pdf
- Häckel, Akvile. “Funktion von Abp1, einem Signal-responsiven Verbindungsglied zwischen Aktinzytoskelett und Membrantransport, in neuronaler Morphologie = Function of Abp1, a signal-responsive linker between actin cytoskeleton and membrane transport, in neuronal morphology”. 2010, https://core.ac.uk/download/224757658.pdf
- Allwood, Blanchoin, Chan, Chaudhry, Christopher J. Staiger, David W. McCurdy, Dixit, et al.. “Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array”. The Rockefeller University Press, 2009, https://core.ac.uk/download/pdf/8267541.pdf
- Allwood, Blanchoin, Chan, Chaudhry, Dixit, Dixit, Dong, et al.. “Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array”. The Rockefeller University Press, 2008, https://core.ac.uk/download/pdf/8267541.pdf
- Dawson, Scott C, Wilson, Katherine L. “Evolution: functional evolution of nuclear structure.”. eScholarship, University of California, 2011, https://core.ac.uk/download/323068606.pdf
- Kressirer, Christine. “Anticancer effects and antimetastatic mechanisms of novel indirubin derivatives”. Ludwig-Maximilians-Universität München, 2010, https://core.ac.uk/download/11031814.pdf
- Ana Alonso, Francisco García-del Portillo. “Hijacking of eukaryotic functions by intracellular bacterial pathogens”. International Microbiology, 2010, https://core.ac.uk/download/159084270.pdf
- Cabanes, D, Carvalho, F, Costa, AC, Sousa, et al.. “Stathmin recruits tubulin to Listeria monocytogenes-induced actin comets and promotes bacterial dissemination”. ‘Springer Science and Business Media LLC’, 2018, https://core.ac.uk/download/302920800.pdf
- Dohle, E., Kirkpatrick, C.J., Li, M., Ma, et al.. “TLR4 stimulation by LPS enhances angiogenesis in a co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells”. ‘Royal College of Obstetricians & Gynaecologists (RCOG)’, 2017, https://core.ac.uk/download/77136222.pdf
- Barnett, Haley. “Influence of Extracellular Cues of Hydrogel Biomaterials on Stem Cell Fate”. Louisiana Tech Digital Commons, 2020, https://core.ac.uk/download/337613671.pdf
- Akin, Orkun, Bieling, Peter, Fletcher, Daniel A, Hansen, et al.. “WH2 and proline-rich domains of WASP-family proteins collaborate to accelerate actin filament elongation.”. eScholarship, University of California, 2017, https://core.ac.uk/download/323080595.pdf
- Fusco, Ludovico. “Computer vision profiling of neurite outgrowth mordphodynamics reveals spatio-temporal modularity of Rho GTPase signaling”. 2014, https://core.ac.uk/download/46604776.pdf
- Serena Paladini, Barbara Truglia, K. Shankar, J. Tuszynski. “Measurement and Characterization of the Electrical Properties of Actin Filaments”. International Journal of Molecular Sciences, 2024, https://www.semanticscholar.org/paper/5ddb33fda65d87286160f27e1fee4c5f592e54cc
- Jianxiao Xing, Ying Wang, A. Peng, Junqin Li, X. Niu, Kaiming Zhang. “The role of actin cytoskeleton CFL1 and ADF/cofilin superfamily in inflammatory response”. Frontiers in Molecular Biosciences, 2024, https://www.semanticscholar.org/paper/d1fe296283f1beeb42105b9732342dc35c29fe73
- ,. “Modelling on the nanomechanics of cytoskeletal filaments”. ‘Swansea University’, 2019, https://core.ac.uk/download/231901832.pdf
- Bakal, Chris, Mak, Michael, Spill, Fabian. “Mechanical and Systems Biology of Cancer”. ‘Elsevier BV’, 2018, https://core.ac.uk/download/185514212.pdf
- Cole, Greg M, Frautschy, Sally A, Ma, Qiu-Lan, Yang, et al.. “PAK in Alzheimer disease, Huntington disease and X-linked mental retardation.”. eScholarship, University of California, 2012, https://core.ac.uk/download/323077488.pdf
- Andrés Benito, Pol, Brullas, Marta, Carmona Murillo, Margarita, Fernández Irigoyen, et al.. “Proteostatic modulation in brain aging without associated Alzheimer’s disease-and age-related neuropathological changes”. Impact Journals, 2023, https://core.ac.uk/download/591790011.pdf
- Masahiro Noji, Yukihiko Sugita, Yosuke Yamazaki, Makito Miyazaki, Yuta Suzuki. “Protein design of two-component tubular assemblies like cytoskeletons”. bioRxiv, 2024, https://www.semanticscholar.org/paper/c758cf4a7649ef7697becac51628e0d61cc05f96
- Agathe Figarol, L. Olive, O. Joubert, L. Ferrari, B. Rihn, F. Sarry, D. Beyssen. “Biological Effects and Applications of Bulk and Surface Acoustic Waves on In Vitro Cultured Mammal Cells: New Insights”. Biomedicines, 2022, https://www.semanticscholar.org/paper/0bc01f2c2e57b0d48a4cd778831bc828e4b5dff0
- Jelena Borovac, Miquel Bosch, Kenichi Okamoto. “Regulation of actin dynamics during structural plasticity of dendritic spines: Signaling messengers and actin-binding proteins”. Molecular and Cellular Neuroscience, 2018, https://doi.org/10.1016/j.mcn.2018.07.001
- Jinsheng Zhu, Aurélien Bailly, Marta Zwiewka, Valpuri Sovero, Martin Di Donato, Pei Ge, Jacqueline Oehri, et al.. “TWISTED DWARF1 Mediates the Action of Auxin Transport Inhibitors on Actin Cytoskeleton Dynamics”. The Plant Cell, 2016, https://doi.org/10.1105/tpc.15.00726
- Jean Moon, Suman Chaudhary, Lorena Rodriguez Martinez, Zhengping Hu, Patricia A. D’Amore. “Endomucin regulates the endothelial cytoskeleton independent of VEGF”. bioRxiv, 2024, https://www.semanticscholar.org/paper/754e5a16c698b7c07f60ba057a066815d3e8c08e
- Bruno A. Cisterna, Kristen Skruber, Makenzie L Jane, Caleb I Camesi, Ivan D Nguyen, Peyton V Warp, Joseph B Black, et al.. “Cytoskeletal adaptation following long-term dysregulation of actomyosin in neuronal processes”. bioRxiv, 2023, https://www.semanticscholar.org/paper/eea3ad237112ecc6a44918a151e7421b3aa2b8c3
Image References:
“Diagram of cellular cytoskeleton components: microfilaments, intermediate filaments, and microtubules..” slcc.pressbooks.pub, 10 January 2025, https://slcc.pressbooks.pub/app/uploads/sites/20/2022/10/Cytoskeleton-1.png
