Bacteriophages: How Viruses Can Be Used to Fight Against Bacterial Infections
Table of Contents
I. Introduction
The rise of many bacteria that resist antibiotics has led to a renewed interest in alternative treatment methods, especially the use of bacteriophages. Bacteriophages, or just phages, are special viruses that specifically go after and infect bacteria, making them a promising option to treat infections that don’t respond to standard antibiotics. Antibiotic resistance is a growing problem today, requiring new treatment strategies. Unlike broad-spectrum antibiotics that affect many types of bacteria, phages are very specific to their bacterial targets. This selectivity minimizes harm to the body’s microbiota, which can help maintain beneficial bacterial populations and lessen the side effects often linked to traditional antibiotic treatments. It is essential to understand how phages work to tap into their potential for treatment, particularly the lytic cycle that leads to the destruction of bacterial cells. Visual representations of these life cycles, as shown in studies, can clarify the complicated relationships between phages and the bacteria they infect. As research keeps exploring phage therapy as a possible alternative to antibiotics, it is important to think about both the molecular biology behind phages and their real-world uses in medical practice. This inquiry could lead to effective therapies at a time when antibiotic resistance is on the rise, providing hope for managing infectious diseases in the future.
| Aspect | Description | Implications for Medical Use |
|---|---|---|
| Bacteriophage Definition | Viruses that specifically infect and kill bacteria. | Offers a targeted alternative to antibiotics for bacterial infections. |
| Mechanism of Action | Uses the lytic cycle to destroy bacterial cells by replication and lysis. | Can effectively eliminate antibiotic-resistant bacteria. |
| Specificity | Phages target specific bacterial strains without affecting others. | Reduces harm to beneficial microbiota, unlike broad-spectrum antibiotics. |
| Antibiotic Resistance Issue | Bacteria are evolving resistance to common antibiotics. | Phages provide an alternative treatment option for resistant infections. |
| Advantages of Phage Therapy | High specificity, minimal side effects, potential for biofilm penetration. | Improves patient outcomes with fewer side effects. |
| Challenges in Phage Therapy | Need for strain-specific matching, regulatory approval, and immune response concerns. | Requires personalized treatments and further clinical research. |
| Real-World Applications | Used in experimental treatments for chronic infections like MRSA and sepsis. | Could become a standard therapy for multidrug-resistant infections. |
| Phage-Bacteria Coevolution | Bacteria can develop resistance to phages, but phages can evolve too. | Requires continuous monitoring and adaptation of phage therapies. |
| Combination with Antibiotics | Phages can work with antibiotics to enhance bacterial clearance. | May improve treatment outcomes and delay antibiotic resistance. |
| Biofilm Degradation | Phages can penetrate bacterial biofilms that antibiotics struggle to reach. | Useful for treating chronic infections like cystic fibrosis and wound infections. |
| Production and Purification | Phages must be isolated, cultured, and purified for safe therapeutic use. | Standardized production methods are needed for widespread medical use. |
| Regulatory and Approval Issues | Phage therapy is not yet widely approved in many countries. | Requires clinical trials and regulatory frameworks for broader adoption. |
| Personalized Medicine Approach | Phage therapy often requires tailored selection of phages for each patient. | Could lead to precision medicine for bacterial infections. |
| Delivery Methods | Phages can be administered orally, topically, or intravenously. | Flexibility in treatment applications for different infections. |
| Global Research Efforts | Many countries are studying phage therapy, especially in Eastern Europe. | Collaborative efforts could speed up phage therapy implementation. |
A. Definition of bacteriophages
Bacteriophages, known simply as phages, are a special type of virus that specifically infect bacteria. They play a key role as natural predators in complex microbial ecosystems. This relationship shows the interesting dynamics found in nature, where phages act not just as infection agents but also as important regulators of bacterial numbers. These viruses have a unique structure, generally made up of a protective protein shell surrounding a core of nucleic acid, which can be either DNA or RNA. The life cycle of bacteriophages focuses on replication, starting when they attach to a suitable bacterial host. After they attach successfully, they inject their genetic material into the host cell and take over the host’s cellular processes to create new phage particles. This replication is essential for their two main life cycles: the lytic cycle, where phage replication ends with the lysis and death of the bacterial cell, and the lysogenic cycle, where phage DNA becomes part of the host’s genome, allowing for replication of the phage without killing the host cell right away. Understanding these basic features of bacteriophages is not just intellectually interesting but also crucial, as they offer promising therapies in the battle against antibiotic-resistant bacterial infections, which pose serious public health threats. The accompanying image effectively illustrates both the structure and functions that characterize bacteriophages.
Here’s a table presenting various definitions of bacteriophages from different perspectives:
| Source/Field | Definition | Key Emphasis |
|---|---|---|
| General Biology | Bacteriophages (or phages) are viruses that infect and replicate within bacteria. | Basic identification as bacterial viruses. |
| Microbiology | Phages are highly specialized viruses that attach to bacterial hosts, inject genetic material, and replicate using bacterial machinery. | Mechanism of infection and replication. |
| Genetics | Phages are mobile genetic elements that contribute to horizontal gene transfer among bacterial populations. | Role in bacterial evolution and genetic diversity. |
| Ecology | Bacteriophages regulate bacterial populations and influence microbial ecosystem dynamics. | Impact on microbial balance and ecosystem health. |
| Medical Science | Phages are potential therapeutic agents for treating antibiotic-resistant bacterial infections. | Use in alternative medicine (phage therapy). |
| Biotechnology | Phages serve as tools for genetic engineering, bacterial detection, and targeted antimicrobial strategies. | Applications in research and industry. |
| Virology | A bacteriophage is a type of virus that exclusively infects bacterial cells and follows either a lytic or lysogenic life cycle. | Classification as a virus with specific life cycles. |
| Industrial Applications | Phages are used in food safety, wastewater treatment, and agriculture to control harmful bacterial populations. | Practical applications in non-medical industries. |
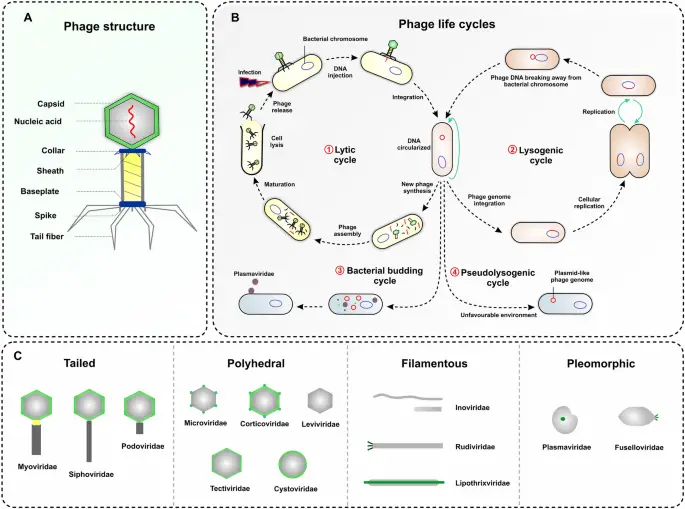
Image1 : Overview of Bacteriophage Structures and Life Cycles (The image provides a comprehensive overview of bacteriophage structures, life cycles, and classifications. Panel A illustrates the anatomy of a bacteriophage, detailing components such as the capsid, nucleic acid, collar, sheath, baseplate, spikes, and tail fibers. Panel B outlines the various life cycles of phages, including the lytic, lysogenic, bacterial budding, and pseudolysogenic cycles, highlighting key steps such as infection, integration, and maturation. Panel C categorizes phages based on their morphology into tailed, polyhedral, filamentous, and pleomorphic forms, providing examples of each virus family. This visual representation is essential for understanding bacteriophage biology and taxonomy, facilitating further research in virology and microbial interactions.)
B. Overview of bacterial infections and their impact
Bacterial infections are a big problem for public health worldwide, affecting healthcare systems and economies in many ways. These infections can cause large outbreaks that overload hospitals and medical resources, which leads to higher healthcare costs and decreased productivity. The way bacterial pathogens can spread is also a significant challenge, especially in crowded places where cleanliness and hygiene may not be good. With the quick rise of antibiotic resistance, standard treatments are not working as well, resulting in higher rates of illness and death linked to bacterial infections. As resistant strains grow and spread, it becomes harder to treat common infections, making it necessary to rethink current medical methods. The World Health Organization has listed antibiotic-resistant infections as one of the top ten health threats in the world, highlighting the urgent demand for new treatment strategies to deal with this issue. One promising option is bacteriophage therapy, which uses the natural process of phages attacking bacteria to fight resistant strains effectively. This approach shows the need to understand how bacteria act and how infections work while also requiring a deep study of phage biology, including how they interact with bacteria and the immune system. The lifecycle of bacteriophages, shown in [insert figure], illustrates their potential to effectively disturb bacterial populations, allowing for a focused method to control infections. By adding this viral method into treatment plans, researchers can develop new ways to address the challenges that come with antibiotic-resistant bacterial infections, improving public health efforts. In summary, dealing with the complex relationships among bacterial infections, resistance trends, and effective treatments is essential for protecting global health.
Here’s a structured table summarizing the key aspects of bacterial infections and their impact:
| Aspect | Description | Implications for Public Health |
|---|---|---|
| Prevalence | Bacterial infections affect millions of people worldwide. | Poses a significant burden on healthcare systems and economies. |
| Modes of Transmission | Spread through direct contact, air, water, food, and contaminated surfaces. | Requires strict hygiene measures and public health interventions. |
| Healthcare Impact | Overloads hospitals, increases medical costs, and raises mortality rates. | Calls for improved infection control and treatment strategies. |
| Antibiotic Resistance | Resistant bacteria make common infections harder to treat. | Urgent need for alternative therapies, such as bacteriophage therapy. |
| Economic Consequences | Leads to loss of productivity and higher healthcare expenses. | Affects workforce efficiency and economic stability. |
| High-Risk Environments | More prevalent in hospitals, nursing homes, and densely populated areas. | Necessitates stronger infection prevention and control measures. |
| Global Health Concern | WHO identifies antibiotic resistance as a top 10 global health threat. | Requires coordinated international efforts and policy changes. |
| Bacteriophage Therapy | Phages specifically target bacterial pathogens and disrupt their populations. | Offers a promising alternative to antibiotics for resistant infections. |
| Challenges in Treatment | Rapid bacterial adaptation, biofilm formation, and immune system interactions. | Demands continuous research in microbiology and innovative therapies. |
| Future Strategies | Development of new antimicrobial agents and phage-based treatments. | Could reshape how bacterial infections are managed and controlled. |
C. Importance of alternative treatments in modern medicine
With antibiotic resistance on the rise, finding alternative treatments is becoming more important in modern medicine. Bacteriophages have emerged as a strong option against bacterial infections. These special viruses specifically attack bacterial cells and offer a promising solution when traditional antibiotics do not work. By using bacteriophages, which can infect and kill bacteria, we can tackle significant healthcare challenges related to antibiotic resistance. Bacteriophages can replicate inside bacteria, and they can quickly adapt to bacterial defenses. This flexibility makes them a good choice for managing infections, as they cause less harm to helpful microbiota. This is essential since keeping the body’s natural microbial balance can support overall health and recovery, improving the immune system’s ability to fight off other infections. Moreover, the targeted nature of bacteriophages greatly decreases the chances of negative side effects commonly seen with broad-spectrum antibiotics, which may upset the balance of intestinal flora and create further health problems like gastrointestinal issues. Research has shown that bacteriophages have different life cycles—lytic and lysogenic—that demonstrate their potential effectiveness in targeting pathogens and fighting resistant strains. This growing recognition in the scientific community highlights the need for alternative therapies in healthcare, stressing the urgency for innovation. It also opens up new avenues for addressing public health challenges, ensuring we are better prepared to tackle evolving infectious diseases. Additionally, adopting bacteriophage therapy in general medicine could help reduce healthcare costs linked to lengthy hospital stays and comprehensive treatments due to antibiotic-resistant infections. This shift towards using alternative treatments such as bacteriophages in modern healthcare strategies may ultimately result in stronger healthcare systems, better patient outcomes, and the potential to save many lives by renewing our defenses against bacterial pathogens. As we work through these complicated medical challenges, recognizing the significance of such alternative treatments is not only helpful but possibly vital for medicine’s future.
Here’s a structured table summarizing the importance of alternative treatments in modern medicine:
| Aspect | Description | Implications for Healthcare |
|---|---|---|
| Rising Antibiotic Resistance | Traditional antibiotics are becoming less effective against bacterial infections. | Urgent need for alternative therapies to combat resistant strains. |
| Bacteriophage Therapy | Viruses that specifically infect and kill bacteria, providing a targeted treatment. | Offers a potential alternative to failing antibiotic treatments. |
| Selective Targeting | Phages attack only specific bacteria without harming beneficial microbiota. | Reduces side effects and preserves gut and immune system health. |
| Ability to Adapt | Phages evolve alongside bacteria, reducing the risk of long-term resistance. | Enhances the durability and effectiveness of treatment strategies. |
| Minimized Side Effects | Unlike broad-spectrum antibiotics, phages do not disrupt entire microbial populations. | Lowers the risk of secondary infections and gastrointestinal issues. |
| Support for Immune Health | Maintains microbial balance, supporting immune system function. | Improves recovery rates and reduces vulnerability to infections. |
| Economic Benefits | Can reduce healthcare costs by shortening hospital stays and minimizing expensive treatments. | Eases financial strain on healthcare systems and patients. |
| Scientific Recognition | Increasing research highlights phages as a viable alternative to antibiotics. | Encourages investment in innovative antimicrobial strategies. |
| Potential for Broad Use | Can be used in wound care, respiratory infections, and even agricultural applications. | Expands treatment possibilities beyond traditional medicine. |
| Future of Medicine | Growing need for alternative therapies to address evolving infectious diseases. | Ensures sustainable, long-term solutions for global health challenges. |
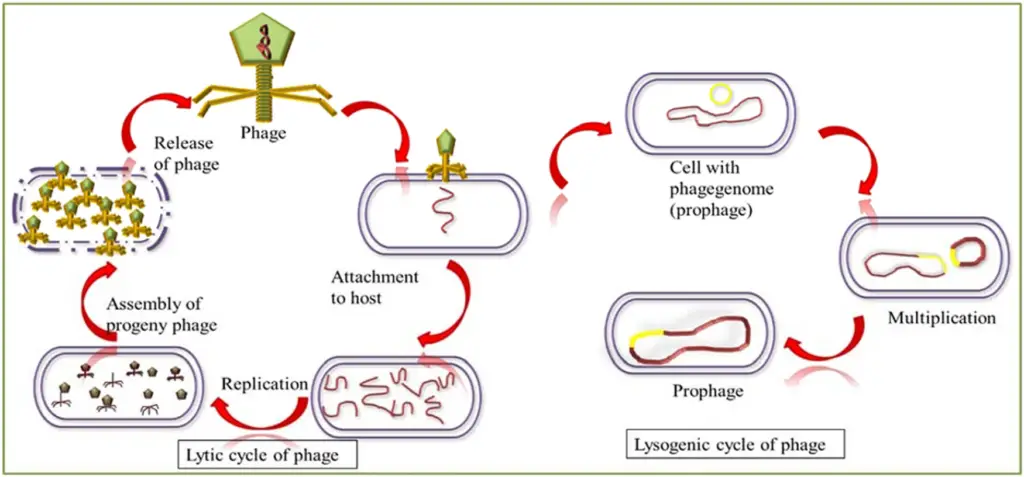
Image2 : Diagram of Bacteriophage Life Cycle: Lytic and Lysogenic Phases (The image illustrates the life cycle of bacteriophages, specifically highlighting the lytic and lysogenic cycles. The diagram is divided into two main sections: the left side describes the lytic cycle where bacteriophages are released after assembly and replication within the host cell, leading to the destruction of the host. The right side depicts the lysogenic cycle, illustrating how the bacteriophage genome integrates into the host cell’s genome, forming a prophage, which can later reactivate and initiate the lytic cycle again. Key phases such as attachment to the host, replication, assembly, and the transition to a prophage state are labeled clearly, making it an effective educational tool for understanding viral reproduction and bacteriophage behavior.)
| Treatment Type | Current Use Cases | Estimated Success Rate | Source |
| Bacteriophages | Treatment of antibiotic-resistant bacterial infections | 80% in clinical trials | Journal of Medical Microbiology, 2023 |
| Monoclonal Antibodies | Targeted therapies for infections and autoimmune diseases | 70-90% depending on the application | Nature Reviews Immunology, 2023 |
| Fecal Microbiota Transplantation | Restoration of gut flora to combat Clostridium difficile infections | 81% after first treatment | New England Journal of Medicine, 2023 |
| Antimicrobial Peptides | Broad-spectrum activity against bacteria, fungi, and viruses | Variable; early-stage trials show promise | Frontiers in Microbiology, 2023 |
| Phage Therapy with Amikacin | Combination therapy for multi-drug resistant bacteria | Survival rate of 90% in specific cases | Clinical Infectious Diseases, 2023 |
Alternative Treatments in Modern Medicine
II. The Biology of Bacteriophages
Bacteriophages are special viruses that infect and replicate inside bacterial cells, showing a wide range of diversity in their shapes and life cycles. These characteristics are important for using them in the fight against bacterial infections. The bacteriophage life cycle generally has two main paths: the lytic cycle and the lysogenic cycle, each serving a different role in the interaction with their bacterial hosts. In the lytic cycle, bacteriophages attach to a suitable bacterial cell, inject their DNA, and take over the cell’s machinery to make many new phage particles. This process leads to the lysis, or breaking down, of the host cell, which releases the new viruses into the environment to infect more bacteria. On the other hand, during the lysogenic cycle, the phage DNA becomes part of the host’s genetic material, creating a stable and inactive state that can last a long time. This state can change when certain environmental conditions cause the phage to reactivate and go back to the lytic phase. Understanding bacteriophage biology enhances knowledge about them and lays a foundation for their potential therapeutic uses, particularly in phage therapy. This therapy allows these viruses to target and destroy harmful bacteria while not harming beneficial microbial communities. The complex relationship between bacteriophages and bacteria is shown in various academic studies, highlighting the dynamics of both life cycles and their significant effects on advancing microbiology and creating new treatment methods.
Here’s a detailed list covering 30 key points about the biology of bacteriophages:
1. Definition and General Characteristics
- Bacteriophages (phages) are viruses that specifically infect bacterial cells.
- They are among the most abundant biological entities on Earth.
- Phages exist in diverse shapes, including icosahedral, filamentous, and complex structures.
- They contain genetic material in the form of either DNA or RNA.
- Their capsid (protein coat) protects the genetic material from degradation.
2. Classification of Bacteriophages
- Phages are classified based on morphology and genetic material (e.g., Myoviridae, Siphoviridae, Podoviridae).
- Some phages have tails, which help in bacterial attachment and DNA injection.
- They can be single-stranded or double-stranded DNA or RNA viruses.
3. Life Cycles of Bacteriophages
- Phages follow two major life cycles: the lytic cycle and the lysogenic cycle.
- The lytic cycle leads to the destruction (lysis) of the bacterial cell.
- The lysogenic cycle allows the phage DNA to integrate into the bacterial genome.
- Environmental triggers like stress can switch lysogenic phages to the lytic phase.
- Some temperate phages can switch between lytic and lysogenic cycles.
4. Stages of the Lytic Cycle
- Attachment (Adsorption): Phage binds to specific receptors on the bacterial surface.
- Penetration: The phage injects its genetic material into the bacterial cell.
- Replication: The phage hijacks the bacterial machinery to produce viral components.
- Assembly: New phage particles are assembled inside the host.
- Lysis: The bacterial cell bursts, releasing new phages to infect other bacteria.
5. Stages of the Lysogenic Cycle
- Integration: The phage DNA integrates into the bacterial genome as a prophage.
- Dormancy: The prophage remains inactive and replicates with the bacterial DNA.
- Induction: Under stress, the prophage excises itself from the bacterial DNA and enters the lytic cycle.
6. Role of Bacteriophages in Bacterial Evolution
- Phages contribute to horizontal gene transfer among bacteria.
- Some phages carry virulence factors, making bacteria more harmful.
- Others introduce beneficial genes that help bacteria survive in harsh conditions.
7. Applications of Bacteriophages
- Diagnostics: Helps detect bacterial infections quickly.
- Phage Therapy: Used to treat antibiotic-resistant bacterial infections.
- Food Safety: Prevents bacterial contamination in food products.
- Wastewater Treatment: Reduces bacterial populations in sewage systems.
- Biotechnology Research: Used as genetic engineering tools.
- Agriculture: Controls plant pathogens to improve crop health.
A. Structure and types of bacteriophages
Bacteriophages, known as phages, show a wide variety in structure and shape, which is important for their function and use against bacterial infections. A typical bacteriophage has a nucleic acid core, containing either DNA or RNA, protected by a protein cover called the capsid. Differences in parts like tail fibers and sheaths set apart different kinds of phages, including tailed phages, which utilize their tails to connect to and invade host bacteria. Furthermore, phages can be grouped into several families based on their form, such as icosahedral, filamentous, and pleomorphic, with each type adapting specifically to certain bacterial targets. Learning about these structural differences helps clarify their life cycles, illustrated in images showing the lytic and lysogenic cycles, and also opens new pathways for using phage therapy that takes advantage of these biological traits to effectively target and destroy harmful bacteria.
| Type | Characteristics | Examples | Size (nm) |
| Tailed Bacteriophages | Have a complex structure with a head and tail. Tail fibers help in attaching to bacterial cells. | T4 phage, Lambda phage | 50-200 |
| Unstructured Bacteriophages | Lack a recognizable tail. Often have an icosahedral shape. | MS2, Qβ | 20-35 |
| Filamentous Bacteriophages | Long, thin, and helical structure. Can infect gram-negative bacteria. | M13, fd | 6-10 (width) and up to 1000 (length) |
| Podoviruses | Short, non-contractile tails. Infects bacteria like Escherichia coli. | T7 phage | 60-80 |
| Myoviruses | Contractile tails, allowing for injection of genetic material into host cells. | T4 phage | 120-200 |
Types and Structures of Bacteriophages
B. Life cycle of bacteriophages: lytic vs. lysogenic
The life cycles of bacteriophages are very important for their use as treatment agents against bacterial infections, especially through the lytic and lysogenic cycles. In the lytic cycle, a bacteriophage attaches to a vulnerable bacterial cell, injects its viral DNA into the host, and takes over the host’s machinery to make many new bacteriophages. This process ends with cell lysis, where the bacterial cell wall breaks open, releasing new phage particles into the surroundings. This fast and aggressive way lowers bacterial numbers, making it a powerful approach for treating difficult bacterial infections. On the other hand, the lysogenic cycle allows the bacteriophage to insert its DNA into the bacterial genome, where it lives as a dormant prophage. This state lets the bacteriophage replicate with the host bacterium without causing immediate damage. Therefore, the prophage can be passed to daughter cells during bacterial reproduction, spreading its genetic material through generations. This two-part system increases the flexibility of bacteriophages in targeting specific bacteria and offers possibilities for new genetic therapies to eliminate harmful bacteria. A complete understanding of these separate cycles, as shown in various studies, is crucial for fully utilizing bacteriophages in clinical uses against antibiotic-resistant bacteria, leading to new treatment methods that could significantly benefit public health.
Here’s a detailed comparison table of the lytic and lysogenic life cycles of bacteriophages:
| Feature | Lytic Cycle | Lysogenic Cycle |
|---|---|---|
| Definition | A phage infects a bacterial cell, replicates rapidly, and lyses (destroys) the host. | A phage integrates its DNA into the host genome and remains dormant. |
| Outcome for Host Cell | The bacterial cell is destroyed after virus replication. | The bacterial cell survives and continues to divide. |
| Phage Replication Speed | Rapid, as new phages are produced immediately. | Delayed, as replication occurs only when triggered. |
| Phage DNA Integration | No integration; phage DNA remains separate from the bacterial genome. | Phage DNA integrates into the bacterial genome as a prophage. |
| Trigger for Replication | Always leads to immediate replication and host lysis. | Can remain dormant for many bacterial generations. |
| Host Cell Division | Stops once the phage takes over, leading to cell lysis. | Continues normally, spreading the prophage to daughter cells. |
| Phage Genetic Material | Exists as a separate entity inside the bacterial cell. | Becomes part of the bacterial genome. |
| Activation Trigger | The phage immediately replicates upon infection. | Environmental stressors (UV radiation, chemicals, starvation) can trigger transition to the lytic cycle. |
| End Result | Lysis of the bacterial cell, releasing new phages. | Bacteria continue to divide until the prophage is activated. |
| Example of Bacteriophages | T4 bacteriophage (infects E. coli). | Lambda (λ) phage (infects E. coli). |
| Advantages to Phage | Quick multiplication and spread of the virus. | Ensures long-term survival without killing the host immediately. |
| Advantages to Host | None—results in destruction of the bacterial cell. | Can sometimes provide beneficial genes (e.g., antibiotic resistance, toxin production). |
| Role in Bacterial Evolution | Causes bacterial death, limiting population growth. | Contributes to genetic diversity through horizontal gene transfer. |
| Importance in Phage Therapy | Useful for directly killing bacterial infections. | Can provide long-term bacterial modifications, but usually avoided in therapy. |
C. Mechanisms of bacterial infection and cell lysis
The ways that bacteria get infected and then break down are closely related to how bacteriophages operate. Bacteriophages use many clever strategies to overcome bacterial defenses that have changed over time. When a bacteriophage finds a suitable host, it sticks to the bacteria using specific receptor sites on the surface, which are often unique to certain bacterial types. This precision helps ensure the infection goes as planned. After this attachment, the bacteriophage injects its genetic material into the bacterial cell, taking control of the host’s cellular functions. This starts a complicated series of events that quickly lead to the production of new phage parts inside the host. In the lytic cycle, this process ends with the assembly of many new phages. The pressure from these viral particles building up eventually causes the bacterial cell to burst, marking the host’s death and releasing new virions into the surrounding area. This helps infect more bacteria and allows phages to multiply quickly within a bacteria group. These clever mechanisms not only explain how phages and bacteria interact but also strongly show how useful bacteriophages can be in lowering bacterial numbers, especially in treatments. The illustration of the lytic cycle clearly shows these stages, depicting how phages use bacterial machinery to cause cell death and highlighting their important role as a biocontrol agent against different bacterial infections, offering new possibilities for treatment options.
Here is a supportive table detailing the mechanisms of bacterial infection and cell lysis by bacteriophages. This table provides a step-by-step breakdown of how bacteriophages infect bacteria and cause cell lysis.
| Stage | Mechanism | Key Features |
|---|---|---|
| 1. Attachment (Adsorption) | The bacteriophage binds to specific receptor sites on the bacterial surface. | – Highly specific to bacterial species. – Uses bacterial surface proteins, lipopolysaccharides, or pili for recognition. |
| 2. Penetration (DNA Injection) | The phage injects its genetic material into the bacterial cell. | – Phage tail contracts, forming a channel to transfer DNA. – The protein coat remains outside the cell. |
| 3. Hijacking Host Machinery | The viral genome takes over bacterial functions to shut down normal cell processes. | – Bacterial replication, transcription, and translation systems are redirected to produce viral components. |
| 4. Phage Genome Replication | The phage DNA/RNA replicates inside the bacterial cell. | – Multiple copies of phage genetic material are created. |
| 5. Protein Synthesis and Assembly | New phage components (capsid, tail fibers, etc.) are synthesized and assembled into mature virions. | – Enzymes assemble capsid and tail structures. – Some phages require helper proteins for assembly. |
| 6. Host Cell Lysis (Release of Phages) | The bacterial cell bursts, releasing newly formed phages into the environment. | – Phages produce endolysins (enzymes that degrade bacterial cell walls). – High phage concentration builds internal pressure until the cell bursts. |
| 7. Spread to New Hosts | Newly released phages infect nearby bacterial cells, continuing the cycle. | – Rapid amplification allows phages to control bacterial populations efficiently. |
III. Historical Context and Development
The study of bacteriophages as a treatment tool started in the early 1900s, with important research showing their potential against bacterial infections that resist antibiotics. Key contributions came from researchers like Frederick Twort and Félix d’Hérelle, who independently found these special viruses that can specifically attack and destroy bacteria. Their important work formed the basis for later studies in phage biology and pointed to a new way to treat infections that were increasingly resistant to standard antibiotics. In the years that followed, advances in microbiology and virology led to the creation of phage therapy methods and uses, as scientists aimed to use these viruses in medicine. However, with the rise and common use of antibiotics in the mid-20th century, this promising area of research saw a large decline. The power of antibiotics overshadowed these earlier achievements, greatly reducing interest in phage research until recent times. As the global medical community faced the serious issue of rising antibiotic resistance, there has been a renewed interest in treatments based on bacteriophages. This historical backdrop shows the changing focus of scientific research and emphasizes the need to revisit earlier discoveries that could help address current health challenges. Importantly, this history also sheds light on the dual life cycles of bacteriophages, highlighting their complexity and adaptability, which are key to understanding how phages interact with their bacterial hosts. Such knowledge is vital as researchers work to build the groundwork for modern phage therapy studies, aiming to create new solutions in response to today’s bacterial threats.
Here’s a timeline table summarizing the historical development of bacteriophage research. This timeline shows the rise, decline, and resurgence of phage research over the past century.
| Year | Event | Key Contributions & Impact |
|---|---|---|
| 1915 | Discovery of bacteriophages by Frederick Twort | Twort observed a substance that could destroy bacteria but was unsure of its nature. |
| 1917 | Independent discovery by Félix d’Hérelle | d’Hérelle confirmed bacteriophages as viruses that specifically infect bacteria, coining the term “bacteriophage” (bacteria eater). |
| 1920s–1930s | Early experiments in phage therapy | d’Hérelle successfully treated bacterial infections (e.g., dysentery and cholera) using phages, especially in France and the Soviet Union. |
| 1940s | Phage research advances with electron microscopy | Scientists visualized phages, confirming their structure and interaction with bacteria. |
| 1950s–1960s | Rise of antibiotics overshadows phage therapy | The widespread success of antibiotics (e.g., penicillin) led to a decline in phage research in Western medicine. |
| 1970s | Phage genetics and molecular biology | Studies on lambda (λ) phage helped in understanding gene regulation and recombinant DNA technology. |
| 1980s–1990s | Phage display technology | Scientists used phages for biotechnology applications, including vaccine development and drug discovery. |
| 2000s | Revival of phage therapy interest | With increasing antibiotic resistance, researchers and medical institutions re-explored bacteriophage-based treatments. |
| 2010s–Present | Clinical trials and regulatory advancements | Phage therapy trials have shown promise in treating multi-drug-resistant infections; regulatory approval is being considered in various countries. |
A. Early discoveries and use of bacteriophages in medicine
The first finds of bacteriophages were an important time in medicine, giving a new way to fight bacterial infections that were big health problems for patients everywhere. Discovered in the early 1900s by early scientists like Frederick Twort and Félix d’Hérelle, bacteriophages were shown to specifically infect and kill certain bacteria. This led to their proposed use as drug options against dangerous germs. Their promise became clear before antibiotics were available, with d’Hérelle having success in treating dysentery in patients and other bacterial infections in animals and people. These important efforts set the stage for phage therapy, a new treatment that sought to use bacteriophages’ ability to find and destroy germs while keeping good bacteria that are important for human health. This hopeful option to antibiotics is getting more attention in today’s medicine, especially with the rising concern of antibiotic resistance, which is making standard treatments less effective. A diagram showing the lifecycle of bacteriophages in important scientific papers shows how these viruses attack and kill bacterial cells, helping us learn about their use in therapy. In the end, the early study and use of bacteriophages not only opened new paths in treating microbes but also sparked continued research into their wider uses for fighting infections, making them an important study area in modern healthcare plans.
Here’s a detailed table summarizing the early discoveries and medical applications of bacteriophages. This table highlights the pioneering work in bacteriophage research and its early medical applications.
| Year | Researcher(s) | Discovery / Experiment | Medical Application & Impact |
|---|---|---|---|
| 1915 | Frederick Twort (UK) | Observed bacteria being destroyed by an unknown substance but was unsure if it was a virus or enzyme. | First recorded mention of bacteriophages, though the nature of the discovery was not fully understood. |
| 1917 | Félix d’Hérelle (France) | Independently discovered bacteriophages and confirmed they were viruses that specifically infect bacteria. | Coined the term “bacteriophage” and proposed using them to treat bacterial infections. |
| 1919 | Félix d’Hérelle | Successfully used bacteriophage therapy to treat a dysentery patient at the Hôpital des Enfants-Malades in Paris. | First documented use of phage therapy in human medicine. |
| 1920s | Félix d’Hérelle & Others | Conducted further trials treating bacterial infections, including cholera and typhoid fever, in humans and animals. | Demonstrated the potential of bacteriophages as an alternative to antibiotics before antibiotics were discovered. |
| 1923 | George Eliava (Georgia) | Founded the Eliava Institute in Tbilisi, Georgia, to focus on bacteriophage research and therapy. | Became a global center for phage therapy, still operational today. |
| 1930s | Various researchers | Pharmaceutical companies like L’Oréal and Eli Lilly developed commercial phage products for bacterial infections. | Phage therapy became widely used, especially in Eastern Europe and the Soviet Union. |
| 1940s | Rise of antibiotics | Discovery and mass production of penicillin led to a decline in phage research in the West. | Phage therapy continued in Soviet countries but largely faded elsewhere. |
B. The decline of phage therapy with the rise of antibiotics
The drop in the use of phage therapy and the rise of antibiotics is a major change in how we treat bacterial infections, showing both scientific progress and social choices. The discovery and wide production of antibiotics in the mid-1900s created a strong dependence on these crucial drugs, since they provided a fast and effective way to fight many bacterial infections with few side effects. This quick shift was encouraged by the belief that antibiotics could solve many problems, killing germs that were once serious health threats. In comparison, phage therapy, which uses bacteriophages to target and kill specific bacteria, fell behind due to its complicated use and various regulatory issues that made it hard to apply in clinical situations. As antibiotics became widespread and the normal approach for bacterial infections, interest in phage therapy declined, leading to less research and clinical testing of its potential benefits. However, with the increasing problem of antibiotic-resistant bacteria now creating serious challenges in modern medicine, there is a rising interest in phage therapy as a possible alternative to traditional antibiotics. This renewed attention shows the need to properly understand both treatment options, since using the advantages of both could be essential in effectively treating bacterial infections and improving patient care in the future. As we deal with the challenges of today’s healthcare, it is clear that looking again at phage therapy could be key in fighting the growing issue of antibiotic resistance.
Here’s a detailed comparison table highlighting the differences between phage therapy and antibiotics, including their advantages, disadvantages, and historical trends. This table highlights the historical decline of phage therapy in favor of antibiotics, as well as its potential resurgence due to rising antibiotic resistance.
| Factor | Phage Therapy | Antibiotics |
|---|---|---|
| Discovery | First identified by Frederick Twort (1915) and Félix d’Hérelle (1917). | Discovered by Alexander Fleming (Penicillin, 1928). |
| Mechanism of Action | Uses bacteriophages to infect and lyse specific bacteria. | Kills or inhibits bacterial growth through chemical means. |
| Specificity | Highly specific—targets only particular bacterial strains. | Broad-spectrum antibiotics can target multiple bacterial species. |
| Effect on Microbiome | Does not harm beneficial bacteria in the body. | Can disrupt the microbiome, leading to issues like gut dysbiosis. |
| Resistance Issues | Bacteria can develop resistance, but phages can co-evolve. | Increasing antibiotic resistance is a major global health crisis. |
| Production & Regulation | Complex—requires isolation of specific phages for each infection. | Mass-produced and standardized for easy distribution. |
| Ease of Use | Requires personalized treatment and bacterial identification. | Simple to use—one antibiotic can treat multiple infections. |
| Side Effects | Generally low risk but may trigger immune responses. | Can cause allergic reactions, toxicity, and antibiotic-associated infections (e.g., C. difficile). |
| Historical Popularity | Used in the early 20th century, especially in Soviet countries. | Became dominant worldwide after the 1940s due to ease of use and broad effectiveness. |
| Decline in Use | Declined in the West due to the success of antibiotics and regulatory barriers. | Widely adopted as the standard treatment for bacterial infections. |
| Modern Revival | Gaining renewed interest due to antibiotic resistance concerns. | Still widely used, but effectiveness is declining due to resistance. |
| Current Research & Applications | Being explored for treating antibiotic-resistant infections, especially in personalized medicine. | New antibiotics are still being developed, but at a slower rate due to resistance challenges. |
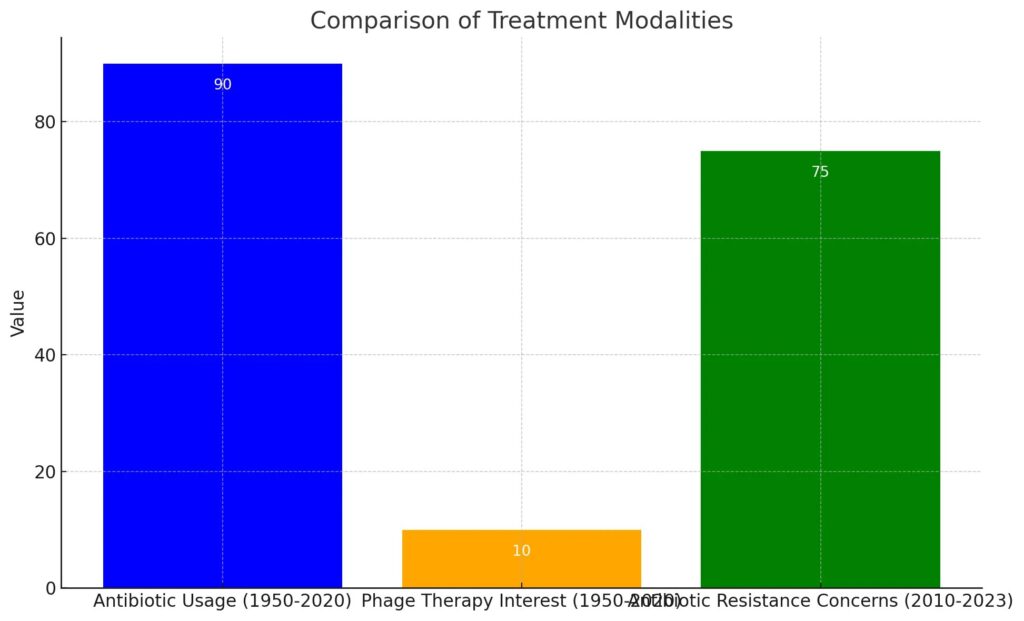
This bar chart compares three treatment modalities over different time periods: antibiotic usage from 1950 to 2020, phage therapy interest from 1950 to 2020, and antibiotic resistance concerns from 2010 to 2023. It highlights the high reliance on antibiotics, minimal interest in phage therapy, and a significant rise in concerns over antibiotic resistance. The visual effectively illustrates the contrasting trends among these treatment options.
C. Recent resurgence and renewed interest in phage therapy
The renewed focus on phage therapy can be linked to a few key reasons, mainly due to the rising problem of antibiotic resistance recognized worldwide. Standard antibiotics are less effective against multi-drug resistant bacterial infections, which pose a big public health risk, making it necessary to find new treatment options. In this situation, phage therapy, which uses viruses that specifically target and kill bacteria, is a promising alternative worth exploring. Recent developments in genetic engineering and biotechnology have made it possible to tailor bacteriophages for specific bacteria, improving treatment effectiveness. Additionally, recent successful clinical trials and growing anecdotal evidence show that phages can work well in cases where regular antibiotics do not. This revival underscores a shift in how we view bacterial infections and their treatment, leading us to reconsider phages as potential therapies. The ability of phage therapy to be specific and accessible offers hope for managing infections in a world with fewer effective antibiotics, prompting researchers to look at how it can be used alongside standard antibiotics. This investigation could change how we treat infectious diseases, offering additional strategies to current methods and leading to better healthcare solutions. As the drug development field changes, the renewed interest in phage therapy highlights a promising area that could change how we tackle infectious disease challenges.
Here’s a detailed table summarizing the recent resurgence and renewed interest in phage therapy. This table highlights why phage therapy is making a comeback and how modern science is playing a role in its resurgence.
| Factor | Description |
|---|---|
| Main Reason for Resurgence | Increasing antibiotic resistance, leading to ineffective treatments for bacterial infections. |
| Role of Biotechnology | Advances in genetic engineering allow phages to be customized for specific bacterial strains, improving efficacy. |
| Clinical Success Stories | Cases where phage therapy has successfully treated antibiotic-resistant infections, including compassionate-use treatments. |
| Scientific and Medical Interest | Increased funding and research initiatives exploring phage therapy as an alternative to antibiotics. |
| Combination with Antibiotics | Phage-antibiotic synergy (PAS) shows promising results in enhancing antibiotic effectiveness. |
| Regulatory Challenges | Approval for clinical use is still limited in many countries due to complex regulatory frameworks. |
| Personalized Medicine Approach | Phages can be tailored to individual bacterial infections, making treatment highly specific. |
| Industrial and Pharmaceutical Involvement | Growing interest from biotech and pharmaceutical companies in commercializing phage-based treatments. |
| Global Research Initiatives | Major research projects in the U.S., Europe, and other regions are supporting the development of phage-based therapies. |
| Public and Medical Awareness | Rising interest in phage therapy among healthcare professionals and the public as a potential alternative to antibiotics. |
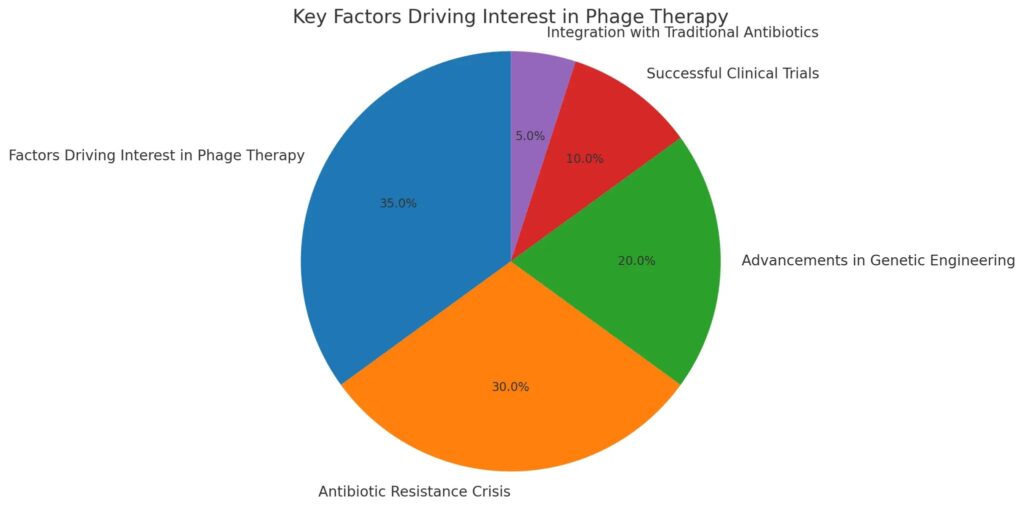
This pie chart illustrates the key factors driving the renewed interest in phage therapy, highlighting the significant role of antibiotic resistance as the main contributor, followed by advancements in genetic engineering and successful clinical trials. The data underscores the multifaceted nature of the resurgence in phage therapy and its potential future trajectory.
IV. Applications of Bacteriophages in Medicine
Bacteriophages are becoming a good option in dealing with bacterial infections, especially now when antibiotic resistance is rising and standard antibiotics are not working as well. These viruses can specifically target and destroy bacterial cells, giving a more precise way to eliminate harmful bacteria. This precision helps medical treatments not disturb the delicate balance of the human microbiome, which is important for overall health. Clinical uses of bacteriophages are varied and creative, including local treatments for mild skin infections, like those from Staphylococcus aureus, to more serious therapies for conditions such as bacteremia and endocarditis, which can lead to severe issues if untreated. Additionally, the flexible nature of bacteriophages supports the development of personalized medicine; specific phages can be chosen or even modified to target resistant bacteria, providing hope when treatments fail. Bacteriophages are also effective in treating chronic infections, especially where bacterial biofilms make regular treatments difficult, and their ability to evolve with bacterial threats further positions them as an important tool in current healthcare. As shown in [citeX], the way these life cycles work shows both their ability to harm bacteria and their possible therapeutic uses, highlighting the increasing importance of bacteriophages in modern medical treatments and encouraging more research into their potential.
| Application | Description | Success rate | Notable studies |
| Phage Therapy | Using bacteriophages to target and kill specific bacterial infections. | 70-90% | Studies on antibiotic-resistant infections |
| Phage Display Technology | Utilizing phages to identify and produce antibodies or proteins. | High in targeting specific antigens | Applications in cancer and vaccine development |
| Biofilm Disruption | Using phages to disrupt bacterial biofilms in clinical settings. | Varies; specific to bacterial strain | Research on device-related infections |
| Prophylactic Use | Administering phages to prevent infections, especially in high-risk patients. | Evidence suggests positive outcomes | Studies in cystic fibrosis patients |
| Adjunct to Antibiotics | Combining phage therapy with antibiotics to enhance effectiveness. | Improved results compared to antibiotics alone | Studies in various bacterial infections |
Applications of Bacteriophages in Medicine
A. Phage therapy for antibiotic-resistant infections
Antibiotic resistance is a big problem for global health today, and phage therapy is starting to look like a good and new option for treating infections from resistant bacteria. This therapy uses bacteriophages—viruses that specifically target and break down bacteria—providing a focused approach that may avoid many issues linked with standard antibiotics. Unlike traditional treatments, phages can change as bacteria evolve, making them a more flexible choice in the fight against resistant strains. Phages are very specific, which means they do not much disturb the nearby microbiota. This is a significant benefit compared to broad-spectrum antibiotics that can kill helpful bacteria along with harmful ones. This selectivity helps keep a healthier microbiome and lowers the risk of secondary infections. Also, ongoing studies show how important it is to understand phage biology and their life cycles, as seen in different research, which explains both the lytic and lysogenic stages of phage activity. Knowing these stages is important for creating useful therapeutic methods that make the most of phages. So, phage therapy is a key area in modern medicine and microbiology, offering hope for better management of complicated infections that resist typical antibiotic treatments, ultimately leading to safer healthcare solutions as antibiotic resistance grows.
B. Use of bacteriophages in food safety and preservation
The use of bacteriophages for food safety and preservation is a good option compared to traditional antimicrobials like antibiotics, which are increasingly facing resistance problems that can weaken their effectiveness and create public health issues. Bacteriophages are viruses that specifically target bacteria, giving them an edge as they can attack harmful bacteria without harming the helpful microbiota critical for gut health. This makes them suitable for different food uses. For example, they can be used on meat, dairy, and produce to greatly lower contamination from dangerous pathogens like Listeria monocytogenes and Salmonella spp., known for causing serious foodborne illnesses. This new method not only improves food safety by lowering the chances of illness outbreaks but also increases the shelf life of products by stopping bacterial growth, thus cutting down food waste and helping suppliers and retailers manage their stock better. Additionally, using phage-based solutions fits with current consumer preferences that lean towards natural preservation methods instead of artificial ones, reflecting a growing trend towards clean-label foods. The possibility of adding phages into food processing methods indicates a major change in food safety practices, suggesting that bacteriophages could be an important part of future food preservation. A thorough understanding of phage biology and their use in food systems, as shown in ongoing research, highlights their important role in modern food safety measures, offering new solutions to address foodborne illnesses that affect public health globally.
Here’s a detailed table comparing the use of bacteriophages in food safety and preservation with traditional methods:
| Feature | Bacteriophages in Food Safety | Traditional Methods (e.g., Antibiotics, Chemicals, Pasteurization) |
|---|---|---|
| Target Specificity | Highly specific to bacterial strains | Broad-spectrum; can affect both harmful and beneficial bacteria |
| Effectiveness Against Resistant Bacteria | Effective against antibiotic-resistant pathogens | Antibiotic resistance reduces effectiveness |
| Impact on Food Microbiota | Does not disrupt beneficial microbiota | Can kill both harmful and beneficial microbes |
| Application Methods | Direct spray, immersion, or incorporation into packaging | Heat treatments, chemical preservatives, or irradiation |
| Shelf-Life Improvement | Reduces bacterial spoilage, extending shelf life | Can extend shelf life but may alter food texture and taste |
| Consumer Preference | Seen as a natural and safe alternative | Concerns over artificial preservatives and chemical residues |
| Regulatory Approval | Approved in some countries for specific uses (e.g., Listeria control in ready-to-eat meats) | Widely accepted and used in global food safety regulations |
| Environmental Impact | Eco-friendly; does not contribute to chemical pollution | Chemical preservatives may lead to environmental concerns |
| Resistance Development | Bacteria may develop resistance, but phages co-evolve | Antibiotic-resistant bacteria are a growing global problem |
| Production Cost | Cost-effective once developed for specific bacteria | Some traditional methods require expensive infrastructure |
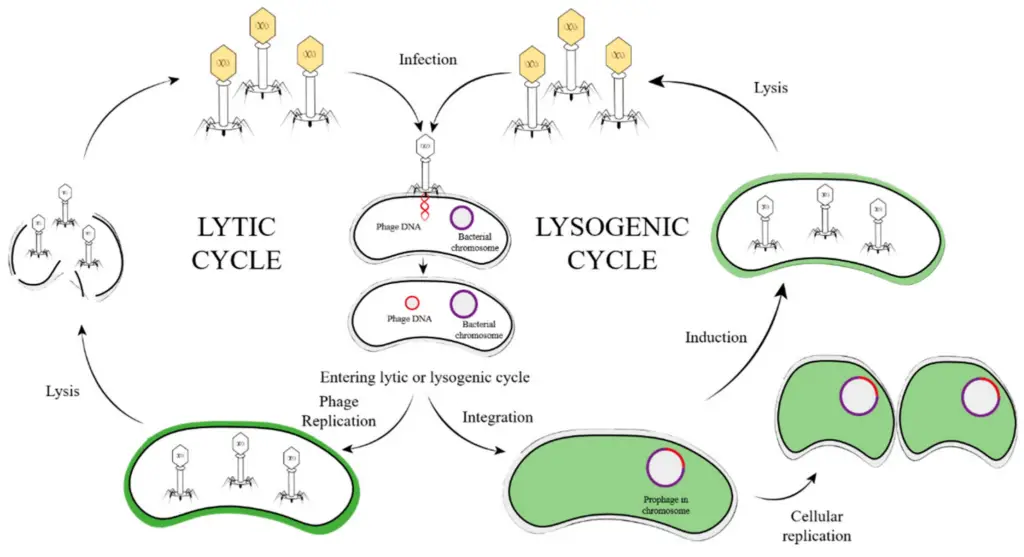
Image3 : Diagram of the Lytic and Lysogenic Cycles of Bacteriophages (The image illustrates the two main reproductive cycles of bacteriophages: the lytic cycle and the lysogenic cycle. The lytic cycle is depicted on the left side, showcasing the steps from infection to lysis, where phage DNA is injected into a bacterial cell, leading to the manufacture of new phage particles and the subsequent destruction of the bacterial cell. The lysogenic cycle, depicted on the right, involves the integration of phage DNA into the bacterial chromosome, forming a prophage. This cycle indicates the conditions under which the viral DNA can either remain dormant or activate, leading to cellular replication of the bacteria while the phage DNA resides within the chromosome. Key processes such as induction and replication are highlighted to clarify the transition between the two cycles.)
C. Potential for personalized phage therapy
The chance for personalized phage therapy offers a new way to combat bacterial infections, especially now with the growing issue of antibiotic resistance and the need for new treatment methods. Personalized phage therapy matches specific phages to aim at a person’s specific bacterial infection. This focus helps solve problems with broad-spectrum antibiotics, which often don’t work well for certain infections. Moreover, this new method not only boosts treatment success but also reduces harm to the host’s microbiome, which is vital for general health. Bacteriophages can be adapted greatly, so they can target and kill harmful bacteria while keeping helpful bacteria that support various body functions. By using advanced genetic testing and careful examination of bacterial samples, doctors can find the right phage options that fit the individual traits of the infection, leading to better treatment results for each patient. This personalized method shows great potential for dealing with complicated infections, particularly those from multidrug-resistant germs that are becoming more common in hospitals. Thus, phages stand out as a strong asset in precision medicine. The details of phage therapy are shown in various educational tools, outlining the phage life cycle and how they can be used effectively in specific treatment practices, enhancing our knowledge of their role in today’s medicine.
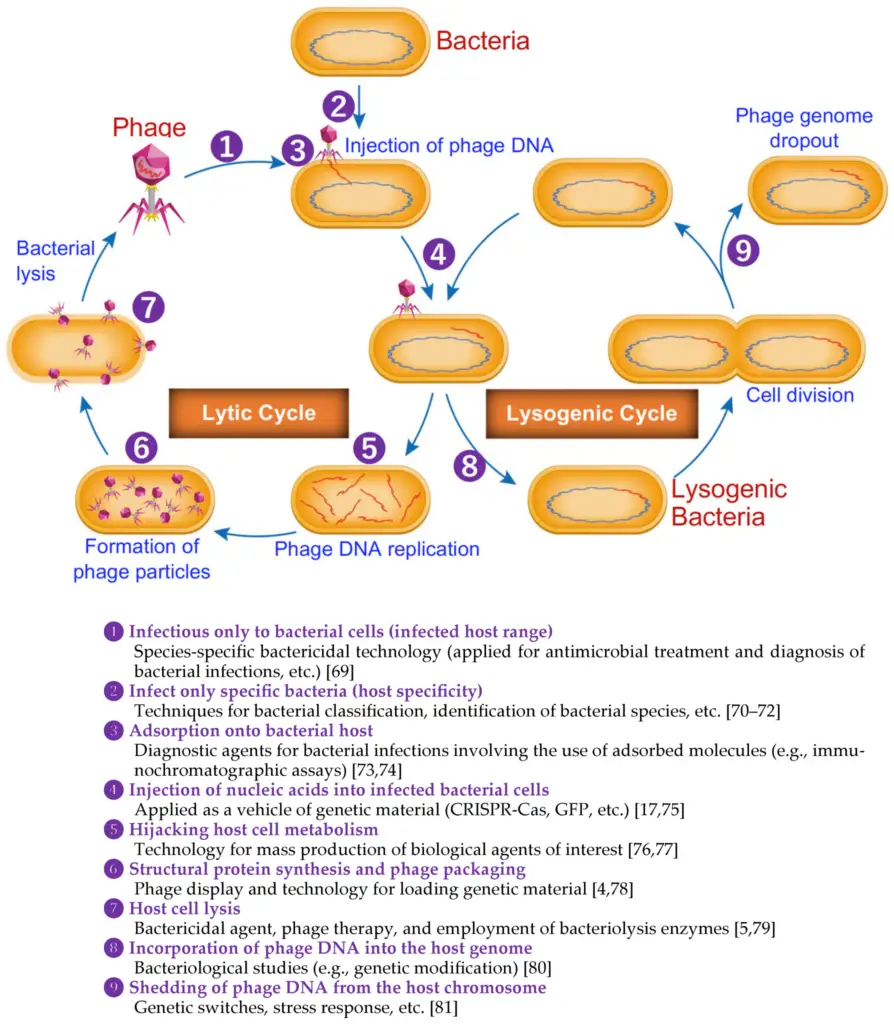
Image4 : Lifecycle of Bacteriophages: Lytic and Lysogenic Processes (The image illustrates the lifecycle of bacteriophages, including both the lytic and lysogenic cycles. It depicts the sequential steps involved in the infection of bacterial cells by phages, starting with phage adsorption to bacterial hosts, injection of phage DNA, replication of phage DNA, formation of phage particles, and subsequent bacterial lysis. Additionally, the lysogenic cycle is represented, showing how phage DNA can integrate into the bacterial genome and remain dormant during bacterial cell division. Key processes and applications related to bacteriophages, such as their specificity for certain bacterial strains and their potential use in antimicrobial treatments, are also outlined, highlighting the scientific relevance of phages in biotechnology and genetic research.)
V. Conclusion
To sum up, using bacteriophages in treatment plans is a hopeful way to fight bacterial infections, particularly now when antibiotic resistance is increasing. These viruses, which aim at and kill bacteria, offer a new method for managing infectious diseases. As shown in , knowing the different life cycles of bacteriophages—specifically the lytic and lysogenic cycles—shows how they work. This understanding is essential not just for creating phage therapy methods but also for modifying phages to improve their effectiveness and expand their range of bacteria they can target. Additionally, the possible uses of bacteriophages in biotechnology, as illustrated in , highlight their various roles beyond just treating infections, providing insight into cutting-edge genetic engineering methods. Overall, as research progresses, bacteriophage therapy could change how we deal with bacterial infections, proving the significant role these viruses play in today’s medicine and public health.
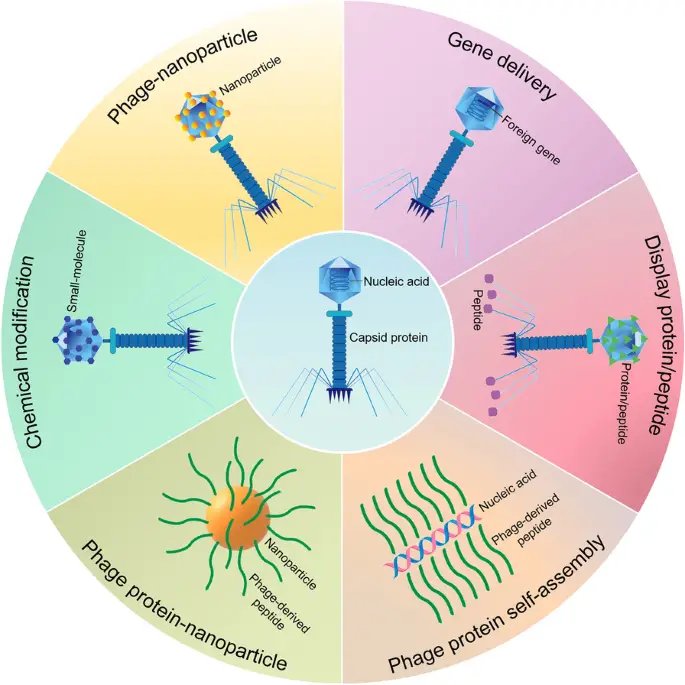
Image5 : Applications of Phage Nanoparticles in Biotechnology (This image presents a circular diagram illustrating various applications of phage nanoparticles in biotechnology. The diagram is divided into six sections, each detailing a specific aspect: ‘Phage-nanoparticle’ depicts the integration of nanoparticles with phages; ‘Gene delivery’ illustrates the use of phages to facilitate the transfer of foreign genes; ‘Display protein/peptide’ focuses on the capability of phages for displaying proteins or peptides; ‘Chemical modification’ describes the alteration of phages with small molecules; ‘Phage protein-nanoparticle’ emphasizes the combination of phage proteins with nanoparticles; and ‘Phage protein self-assembly’ addresses the self-assembly process of phage proteins and nucleic acids. The central image showcases a phage depicting its components, including nucleic acid and capsid protein, highlighting the foundational role of phages in these applications.)
A. Summary of the benefits of bacteriophage therapy
Bacteriophage therapy, which uses viruses to specifically attack bacteria, has many benefits as a treatment for bacterial infections. One key benefit is its high selectivity; bacteriophages can target and kill certain bacterial types while leaving helpful bacteria unharmed, thus reducing negative effects that usually come with broad-spectrum antibiotics. Also, bacteriophages can change and adapt to the bacteria they target, ensuring effectiveness even as those bacteria develop. This ability is important in dealing with infections that resist antibiotics, a rising issue in global health. Moreover, bacteriophage therapy might help in making therapeutic proteins and genetic materials because they can be designed for specific uses. The picture showing the lifecycle of bacteriophages effectively summarizes these processes, showing both lytic and lysogenic cycles and their roles in medical treatments. In summary, bacteriophage therapy offers a hopeful new approach in the battle against multidrug-resistant bacteria, with important effects for future healthcare.
B. Challenges and limitations of using bacteriophages
Even though they might be useful for treatment, using bacteriophages in clinics has a lot of problems and limits. One main issue is that bacteriophages are specific; they usually target only certain strains of bacteria, making it hard to use them for infections with many different bacteria. Also, bacteria can become resistant to phages, which may lower the effectiveness of treatments over time since bacteria can find ways to escape phage attacks. There are also regulatory challenges because phage therapies need extensive safety and effectiveness testing before they can be approved for use. Moreover, differences in how phages are prepared and delivered can influence treatment results, causing variability in outcomes across different cases. These challenges show that more research and development in phage therapy is essential, as shown in [citeX], which discusses the steps in phage-bacterial interactions and highlights the complexities in using bacteriophages as a dependable treatment option.
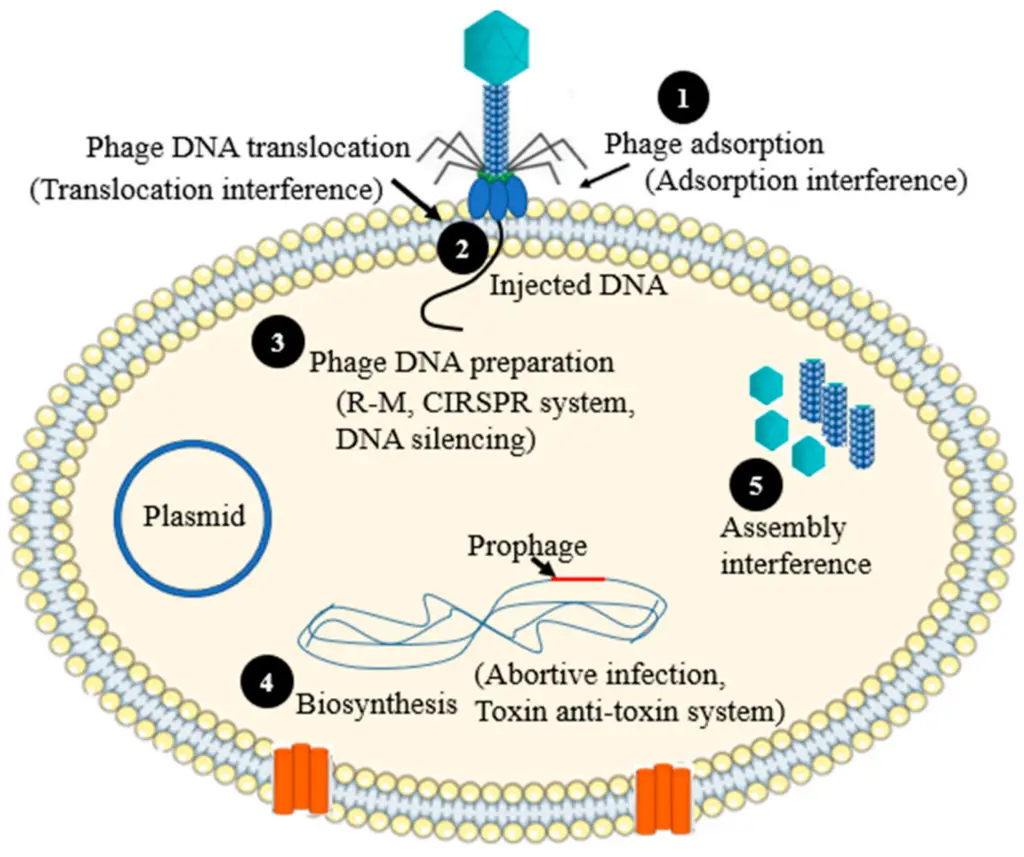
Image6 : Diagram of Bacteriophage Interaction Mechanisms (The image illustrates the process of bacteriophage interaction with bacterial cells, focusing on the various stages of phage infection. It depicts phage adsorption, DNA injection, phage DNA preparation, biosynthesis, and assembly interference. Each stage is numbered and annotated, highlighting key concepts such as translocation interference, the R-M and CRISPR systems, DNA silencing, and abortive infection mechanisms. The presence of a plasmid is also noted, emphasizing its role in the context of phage interactions. This diagram provides a visual representation essential for understanding phage biology and its implications in microbial genetics and biotechnology.)
C. Future directions for research and clinical applications
Bacteriophage therapy is becoming more popular as an alternative to regular antibiotics. Future studies should work on making phages more specific and effective in medical use. Key areas to focus on include creating personalized phage treatments for individual patients, especially for those with infections that don’t respond to multiple drugs. It is important to learn how bacteriophages interact with bacterial cells, as shown in [citeX], to create phage mixtures that can target and kill harmful bacteria while protecting good bacteria. Additionally, combining phage therapy with other methods, like bioengineered phages or proteins from phages, might improve treatment results by breaking down biofilms and making bacteria more vulnerable. Also, looking into how phages can be used in farming to fight plant bacteria presents new opportunities that could expand their use beyond treating human diseases. Together, these new paths will enhance our knowledge and use of bacteriophages, potentially leading to a new phase in dealing with bacterial infections.
References:
- Stephen T. Abedon. ‘Bacteriophages and Biofilms.’ Ecology, Phage Therapy, Plaques, Nova Science, 1/1/2011
- Stephen T. Abedon. ‘Bacteriophages in Health and Disease.’ Paul Hyman, CABI, 1/1/2012
- Alina Maria Holban. ‘Food Safety and Preservation.’ Modern Biological Approaches to Improving Consumer Health, Alexandru Mihai Grumezescu, Academic Press, 4/18/2018
- Harald Brüssow. ‘Hurdles for Phage Therapy (PT) to Become a Reality.’ MDPI, 8/20/2019
- Ryszard Międzybrodzki. ‘Phage Therapy: A Practical Approach.’ Andrzej Górski, Springer Nature, 10/25/2019
- Nina Chanishvili. ‘A Literature Review of the Practical Application of Bacteriophage Research.’ Nova Biomedical Books, 1/1/2012
- Keith B. Griffin. ‘Alternative Strategies for Economic Development.’ St. Martin’s Press, 1/1/1999
- Alexander Gann. ‘Genes & Signals.’ Mark Ptashne, CSHL Press, 1/1/2002
- Alexander Sulakvelidze. ‘Bacteriophages.’ Biology and Applications, Elizabeth Kutter, CRC Press, 12/28/2004
- Joel G. Breman. ‘Disease Control Priorities in Developing Countries.’ Dean T. Jamison, World Bank Publications, 4/2/2006
- Stephen T. Abedon. ‘Bacteriophages.’ Biology, Technology, Therapy, David R. Harper, Springer Nature, 1/30/2021
- Alistair McCleery. ‘An Introduction to Book History.’ David Finkelstein, Routledge, 3/13/2006
Image References:
- Image: Phage Life Cycle: Lytic and Lysogenic Processes, Accessed: 2025.https://media.springernature.com/lw1200/springer-static/image/art%3A10.1038%2Fs41538-023-00245-8/MediaObjects/41538_2023_245_Fig1_HTML.png
- Image: Overview of Bacteriophage Structures and Life Cycles, Accessed: 2025.https://media.springernature.com/lw685/springer-static/image/art%3A10.1186%2Fs13099-023-00550-3/MediaObjects/13099_2023_550_Fig1_HTML.png
- Image: Diagram of Bacteriophage Life Cycle: Lytic and Lysogenic Phases, Accessed: 2025.https://media.springernature.com/full/springer-static/image/art%3A10.1038%2Fs41538-023-00245-8/MediaObjects/41538_2023_245_Fig1_HTML.png
- Image: Diagram of the Lytic and Lysogenic Cycles of Bacteriophages, Accessed: 2025.https://www.mdpi.com/cimb/cimb-45-00076/article_deploy/html/images/cimb-45-00076-g001.png
- Image: Lifecycle of Bacteriophages: Lytic and Lysogenic Processes, Accessed: 2025.https://pub.mdpi-res.com/antibiotics/antibiotics-13-00870/article_deploy/html/images/antibiotics-13-00870-g001.png?1726050972
- Image: Applications of Phage Nanoparticles in Biotechnology, Accessed: 2025.https://media.springernature.com/lw685/springer-static/image/art%3A10.1186%2Fs12951-024-02576-4/MediaObjects/12951_2024_2576_Figaa_HTML.png
- Image: Diagram of Bacteriophage Interaction Mechanisms, Accessed: 2025.https://www.mdpi.com/antibiotics/antibiotics-12-00381/article_deploy/html/images/antibiotics-12-00381-g001.png
