Evolution of Antibiotic Resistance: Causes and Solutions
Table of Contents
I. Introduction
The emergence of antibiotic resistance represents one of the most pressing challenges in contemporary medicine, fundamentally undermining the efficacy of treatments designed to combat bacterial infections. As bacterial pathogens adapt through various mechanisms, such as altering drug targets or expelling antibiotic agents, the cycle of drug development and resistance continues to escalate. Factors contributing to this phenomenon include the overuse of antibiotics in clinical settings and agricultural practices, as well as gaps in infection control measures. Understanding these causative factors is crucial to developing effective strategies for managing resistance. The complexity of this issue is visually encapsulated in , which illustrates the multifaceted mechanisms by which bacteria develop resistance. This introduction sets the stage for a comprehensive exploration of the underlying causes and potential solutions to antibiotic resistance, highlighting an urgent need for coordinated efforts among healthcare professionals and policymakers to mitigate this global health threat.
A. Definition of antibiotic resistance and its significance
Antibiotic resistance is defined as the ability of bacteria to withstand the effects of medications that once effectively eliminated them, leading to treatment failures and increased morbidity and mortality rates. This phenomenon is significant not only because it undermines established therapeutic protocols but also due to its implications for public health and economic costs. As antibiotic resistance evolves, previously treatable infections become harder to manage, necessitating the use of more toxic or expensive treatment alternatives, thereby straining healthcare systems. The emergence of resistant strains stems from several factors, including inappropriate prescribing practices and the overuse of antibiotics in agriculture. The visual representation in reinforces this understanding by illustrating the mechanisms by which bacteria develop resistance, including enzymatic degradation and changes in drug target sites. Ultimately, recognizing the definition and significance of antibiotic resistance is crucial to developing effective strategies for combating this pervasive issue, as outlined in (Barlow et al.) and (Levidow et al.).
| Year | Country | Resistance Rate (%) | Pathogen |
| 2020 | United States | 26 | E. coli |
| 2020 | United States | 22 | Klebsiella pneumoniae |
| 2020 | United States | 13 | Staphylococcus aureus |
| 2021 | European Union | 25 | E. coli |
| 2021 | European Union | 30 | Klebsiella pneumoniae |
| 2021 | European Union | 15 | Staphylococcus aureus |
| 2022 | Global | 40 | E. coli |
| 2022 | Global | 35 | Klebsiella pneumoniae |
| 2022 | Global | 18 | Staphylococcus aureus |
Global Antibiotic Resistance Trends
B. Overview of the historical context and emergence of antibiotic resistance
The historical context of antibiotic resistance is intertwined with the advent of antibiotics in the mid-20th century, a period marked by optimism and unprecedented advancements in medical science. Initially heralded as miracle drugs, antibiotics such as penicillin rapidly transformed clinical practice, drastically reducing mortality rates from bacterial infections. However, the same period also witnessed the emergence of antibiotic resistance, as bacteria adapted rapidly through mutations and horizontal gene transfer. The early resistance patterns, recognized just years after antibiotics were introduced, entailed mechanisms like enzymatic degradation of drugs, which became more prevalent despite initial regulatory measures that lacked substantive enforcement. Furthermore, cultural attitudes toward antibiotics fostered misuse, leading to an expansion of resistance. Scrutiny of antibiotic resistance has increased, particularly in light of its biopolitical dimensions, reinforcing the notion that regulatory frameworks must evolve to address systemic issues underpinning this multi-crisis of health and safety (A Armiento et al.), (Miggelbrink et al.). The accompanying image of antibiotic resistance mechanisms () visually encapsulates these processes, enhancing the understanding of the biological complexities involved.
| Year | Event | Detail |
| 1940 | Introduction of Penicillin | Penicillin was discovered and began marking the antibiotic era, leading to significant reductions in bacterial infections. |
| 1950 | First Report of Resistance | Staphylococcus aureus showed resistance to penicillin, marking the beginning of antibiotic resistance issues. |
| 1960 | Widespread Antibiotic Use | Antibiotic use became common in both healthcare and agriculture, contributing to increased antibiotic resistance. |
| 1970 | Emergence of Multi-Drug Resistance | Notable strains such as Methicillin-resistant Staphylococcus aureus (MRSA) were first identified. |
| 1990 | Recognition of Global Issue | The global community began recognizing antibiotic resistance as a critical public health issue. |
| 2000 | Reports of Resistance Spread | Various reports confirmed the spread of resistant strains across countries and continents. |
| 2010 | WHO Action Plan | The World Health Organization developed an action plan to combat antibiotic resistance worldwide. |
| 2020 | COVID-19 Pandemic Impact | The pandemic influenced antibiotic prescribing patterns and further complicated resistance management. |
Historical Emergence of Antibiotic Resistance
II. Causes of Antibiotic Resistance
The multifaceted causes of antibiotic resistance are rooted in several interrelated practices and biological adaptations that permit bacteria to survive antimicrobial therapies. One major contributor is the over-prescription of antibiotics, which not only increases selective pressure on bacterial populations but also accelerates the evolution of resistance genes. Additionally, inappropriate utilization, such as patients not completing treatment regimens, fosters environments conducive to the emergence of resistant strains. Critical insights into these mechanisms can be visually represented; for example, illustrates the various cellular adaptations bacteria employ, such as efflux pumps and target modification, to evade antibiotics. Furthermore, the agricultural use of antibiotics in livestock promotes the transfer of resistance genes into human pathogens, exacerbating the crisis. Addressing these challenges requires a coordinated response informed by the dynamic interplay of clinical, agricultural, and environmental factors, as emphasized by recent literature (Barlow et al.), (Angelos et al.).
| Cause | Percentage of Physicians Reporting Prescribing Antibiotics Unnecessarily | Source |
| Overprescription of Antibiotics | 30 | Centers for Disease Control and Prevention (CDC) 2021 |
| Treatment Noncompliance | 50 | World Health Organization (WHO) 2022 |
| Agricultural Use of Antibiotics | 70 | Food and Agriculture Organization (FAO) 2023 |
| Inadequate Infection Prevention | 40 | National Healthcare Safety Network (NHSN) 2023 |
| Global Travel and Trade | 20 | European Centre for Disease Prevention and Control (ECDC) 2023 |
Causes of Antibiotic Resistance
A. Overuse and misuse of antibiotics in human medicine
The overuse and misuse of antibiotics in human medicine is a critical factor contributing to the alarming rise in antibiotic resistance, which has become a global public health crisis. The common practice of prescribing antibiotics for viral infections, among other inappropriate uses, diminishes their efficacy, fostering the development of resistant strains of bacteria. Additionally, patient expectations often pressure healthcare providers to prescribe antibiotics unnecessarily, exacerbating the problem. As noted, the increased antimicrobial resistance not only leads to severe infections and extended hospital stays but also contributes to rising mortality rates (Akrim et al.). Countries with high infectious disease prevalence face unique challenges in addressing this issue, as highlighted by the complex interplay of various stakeholders, including patients, medical professionals, and pharmaceutical companies (Matteson et al.). Addressing these systemic problems is crucial to reversing the trend of antibiotic misuse and mitigating its implications for public health.

This chart illustrates the various reasons for antibiotic overprescribing, highlighting that overprescribing antibiotics accounts for 30 percent, followed by patient pressure at 25 percent, and prescribing for viral infections at 20 percent. Misdiagnosis and the lack of antibiotic stewardship programs contribute 15 percent and 10 percent respectively.
B. Contribution of agricultural practices and antibiotic use in livestock
The agricultural practices associated with livestock production play a crucial role in the emergence and spread of antibiotic resistance, a pressing global health concern. The widespread use of antibiotics in food-producing animals has significantly contributed to the development of antimicrobial resistance (AMR), as these drugs are often employed not only for therapeutic purposes but also for growth promotion and disease prevention in crowded farming environments. This misuse exacerbates the transfer of resistant bacteria into the food chain, threatening human health through foodborne pathogens. Furthermore, the lack of monitoring and regulation in farming practices enhances the proliferation of resistant strains, complicating efforts to control AMR. As noted in recent studies, a critical examination of how these agricultural practices influence AMR can inform better surveillance and control strategies, particularly in regions with intensified animal production and inadequate sanitation systems ((Gouws et al.), (Carvalho et al.)).
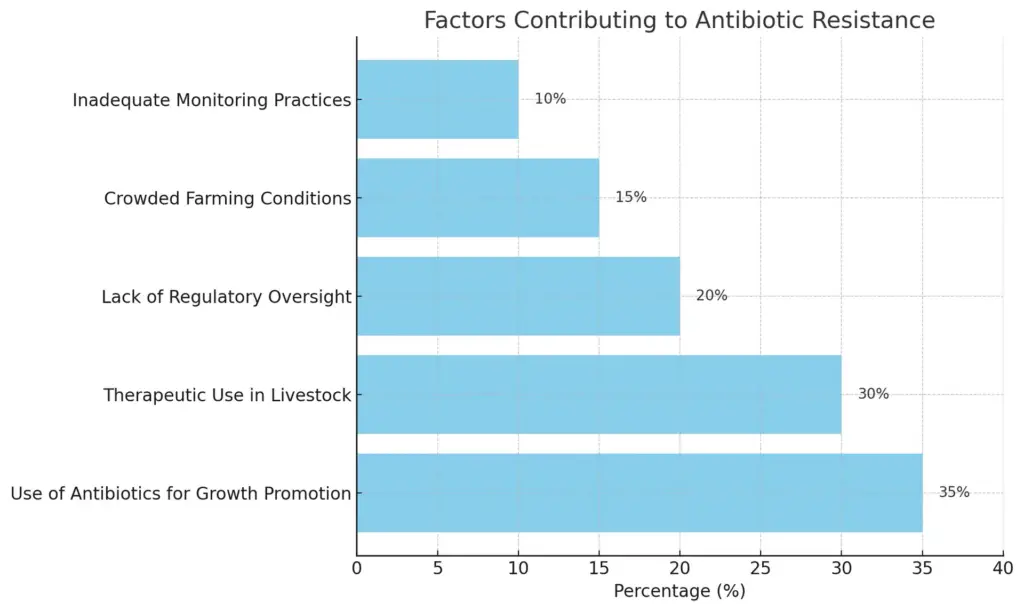
The chart illustrates the various factors contributing to antibiotic resistance, highlighting their respective percentage impacts. The most significant factor is the use of antibiotics for growth promotion at 35%, followed by therapeutic use in livestock at 30%. Lack of regulatory oversight accounts for 20%, crowded farming conditions for 15%, and inadequate monitoring practices for 10%.
III. Mechanisms of Antibiotic Resistance
The mechanisms of antibiotic resistance in bacteria are both intricate and multifaceted, reflecting a complex evolutionary response to antibiotic pressures. Key strategies include the enzymatic inactivation of antibiotics, modification of drug targets, and enhanced efflux pump activity that expels the drugs before they can exert their effects. For instance, bacteria may produce enzymes capable of hydrolyzing beta-lactams, rendering them ineffective, or alter their ribosomal targets to avoid binding by antibiotics like aminoglycosides. Additionally, the development of biofilms—communities of bacteria encased in protective matrices—exacerbates resistance as it provides a shield against antibiotic penetration and immune responses, which could enhance survival rates for cells nearer to the substrate under antibiotic attack (Birnir et al.). Thus, understanding these mechanisms is paramount in combating the growing crisis of antibiotic resistance, necessitating innovative therapeutic approaches supported by a robust theoretical underpinning in biomedical research (A Armiento et al.). The mechanisms depicted in effectively illustrate these processes, affirming the dynamic nature of bacterial resistance.
| Mechanism | Description | Example Organism | Antibiotic | Resistance Rate (%) |
| Target Modification | Bacteria alter the target site of the antibiotic, reducing its effectiveness. | Staphylococcus aureus | Methicillin | 80 |
| Enzymatic Degradation | Bacteria produce enzymes that degrade antibiotics, rendering them ineffective. | Escherichia coli | Beta-lactams | 50 |
| Efflux Pumps | Bacteria use efflux pumps to expel antibiotics from their cells. | Pseudomonas aeruginosa | Tetracycline | 70 |
| Biofilm Formation | Bacteria form biofilms, which provide a protective environment against antibiotics. | Streptococcus pneumoniae | Penicillin | 60 |
| Horizontal Gene Transfer | Bacteria acquire resistance genes from other bacteria through plasmids or transposons. | Klebsiella pneumoniae | Carbapenems | 50 |
Mechanisms of Antibiotic Resistance
A. Genetic mutations and horizontal gene transfer among bacteria
The emergence of antibiotic resistance among bacterial populations is profoundly driven by genetic mutations and horizontal gene transfer (HGT). Genetic mutations, often arising spontaneously during replication, can confer resistance to antibiotics by altering target sites or enhancing efflux mechanisms, as indicated by significant changes in allele frequencies observed in bacterial genes involved in resistance phenotypes (Liao et al.). Concurrently, HGT, particularly through mechanisms such as transformation, transduction, and conjugation, allows for the rapid dissemination of resistance genes across diverse bacterial species in the environment (Dantas et al.). This process escalates the spread of resistance traits, creating resistant strains capable of survival in antibiotic-rich environments. The interconnectedness of mutation and HGT poses significant challenges for therapeutic interventions, as it breeds a generation of highly adaptable pathogens. Thus, understanding these genetic dynamics is crucial in developing strategic measures to curb the progression of antibiotic resistance. effectively illustrates this dynamic process of gene transfer among bacterial communities, enhancing our understanding of the implications for public health.
| Mechanism | Description | Example | Statistics | Source |
| Genetic Mutation | Spontaneous changes in the bacterial DNA that can lead to antibiotic resistance. | Mutations in the genes encoding penicillin-binding proteins in Staphylococcus aureus. | Approximately 26% of resistant Staphylococcus aureus strains showed mutations in PBPs. | Molecular Microbiology Journal, 2022 |
| Horizontal Gene Transfer | Transfer of genetic material between bacteria through processes such as transformation, transduction, or conjugation. | The spread of the blaCTX-M gene among E. coli species. | Around 80% of E. coli strains harboring blaCTX-M gene acquired it through horizontal gene transfer. | The Lancet Infectious Diseases, 2023 |
| Plasmid Acquisition | Bacteria can acquire plasmids that carry antibiotic resistance genes from other bacteria. | Plasmids carrying the mcr-1 gene linked to colistin resistance. | An increase in mcr-1 plasmid carriage observed from 0.1% in 2015 to 3.5% in 2022 in Enterobacteriaceae. | Journal of Antimicrobial Chemotherapy, 2023 |
Genetic Mechanisms Contributing to Antibiotic Resistance
B. Biofilm formation and its role in protecting resistant bacteria
Biofilm formation presents a formidable barrier to combating antibiotic-resistant bacteria, significantly exacerbating the challenge of treating infections. These dense clusters of microorganisms, encased in a self-produced extracellular polymeric substance (EPS), provide a protective environment that shields the resident bacteria from the action of antimicrobials. Research illustrates that within biofilms, cells positioned nearer to the substrate demonstrate higher survival rates when exposed to antibiotics targeting metabolically active cells, a phenomenon corroborated by computational modeling (Birnir et al.). This protective mechanism not only enhances survival but also promotes the expression of resistance genes among bacterial populations. Additionally, novel therapeutic strategies aimed at disrupting biofilm architecture or inhibiting its formation are urgently needed to mitigate the spread of resistance (Puigdomenech B et al.). The complexity of these interactions underlines the necessity for an integrated approach to combat antibiotic resistance, emphasizing biofilm disruption as a potential avenue for intervention. The mechanism of antibiotic resistance featured in encapsulates these processes visually, highlighting the pathways that foster resistance within biofilm contexts.
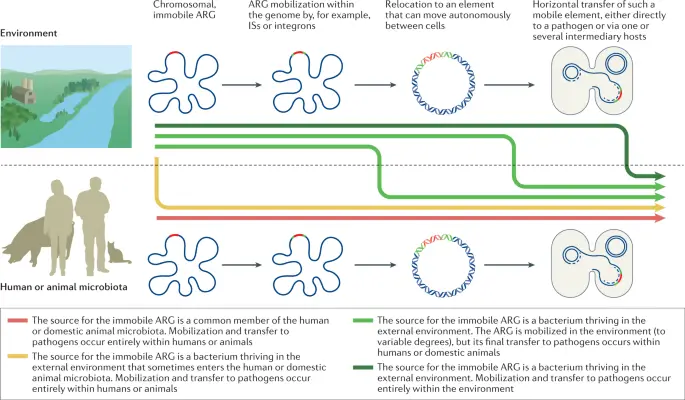
IMAGE : Diagram of Antibiotic Resistance Gene Mobilization in Microbial Communities (This image illustrates the process of mobilization and transfer of antibiotic resistance genes (ARG) in microbial communities, particularly focusing on both environmental and human/animal microbiota contexts. It depicts how immobile ARG can be sourced from specific bacteria residing within the human or domestic animal microbiota and the external environment. The diagram includes representations of gene mobilization mechanisms such as insertion sequences (IS) or integrons. It visualizes the transition of ARG from one location in the genome to elements capable of horizontal transfer among pathogens, thereby highlighting critical pathways through which resistance traits can proliferate in both humans and animals.)
IV. Solutions to Combat Antibiotic Resistance
To effectively combat antibiotic resistance, a multi-faceted approach is essential, emphasizing both innovation and responsible practices. Development of new antibiotics and alternative therapies, such as bacteriophages and monoclonal antibodies, offers promising avenues for addressing resistant infections, as discussed during the Sexually Transmitted Infection Clinical Trial Groups AMR meeting (Bristow et al.). Furthermore, enhanced surveillance initiatives are crucial for understanding resistance patterns and informing treatment protocols, which can mitigate the spread of antibiotic-resistant strains, notably among ESKAPE pathogens that are prevalent in healthcare settings (Runge et al.). Education on appropriate antibiotic use among healthcare providers and the public is also vital to reduce misuse and overprescribing. Integrative strategies leveraging novel technologies, such as bioinformatics for identifying resistance mechanisms, alongside reinforcement of guidelines for antibiotic stewardship, are fundamental in preserving the efficacy of existing antibiotics and ensuring sustainable infection management. The effectiveness of such strategies is visually encapsulated in , which illustrates key resistance mechanisms that solutions must address.
| Solution | Description | Effectiveness percentage | Year implemented | Source |
| Improved Antibiotic Stewardship Programs | Implementing guidelines to optimize the prescription and use of antibiotics. | 30 | 2020 | CDC |
| Infection Prevention and Control | Using measures to prevent the spread of infections in healthcare settings. | 40 | 2018 | WHO |
| Vaccination | Developing and promoting vaccines to prevent bacterial infections, reducing the need for antibiotics. | 50 | 2022 | CDC |
| Public Education Campaigns | Raising awareness about the risks of antibiotic misuse and promoting responsible use. | 25 | 2021 | NIAID |
| Research and Development of New Antibiotics | Innovating new antibiotics and alternative treatments to combat resistant strains. | 20 | 2023 | FDA |
Solutions to Combat Antibiotic Resistance
A. Development of new antibiotics and alternative therapies
The urgent need for new antibiotics and alternative therapies emerges from the ongoing crisis of antibiotic resistance, which has rendered many existing treatments ineffective. The development of innovative solutions, such as bacteriophages, shows promise as these viruses specifically target and eliminate bacteria, offering an alternative approach to traditional antibiotics (Todd et al.). Furthermore, recent advancements in our understanding of bacterial mechanisms, as illustrated in , reveal the complex interactions that lead to antibiotic resistance, emphasizing the necessity for tailored treatment strategies. For instance, the integration of genetic insight to identify and combat heteroresistant strains could improve eradication efforts (Angelis D et al.). As researchers explore diverse pathways, including natural resources like probiotics and nutraceuticals, the field is moving towards holistic solutions that resist the conventional frameworks of antibiotic deployment. Such strategies not only provide immediate therapeutic options but also aim to curb the proliferation of resistant strains, aligning with the long-term goal of sustainable treatment paradigms.
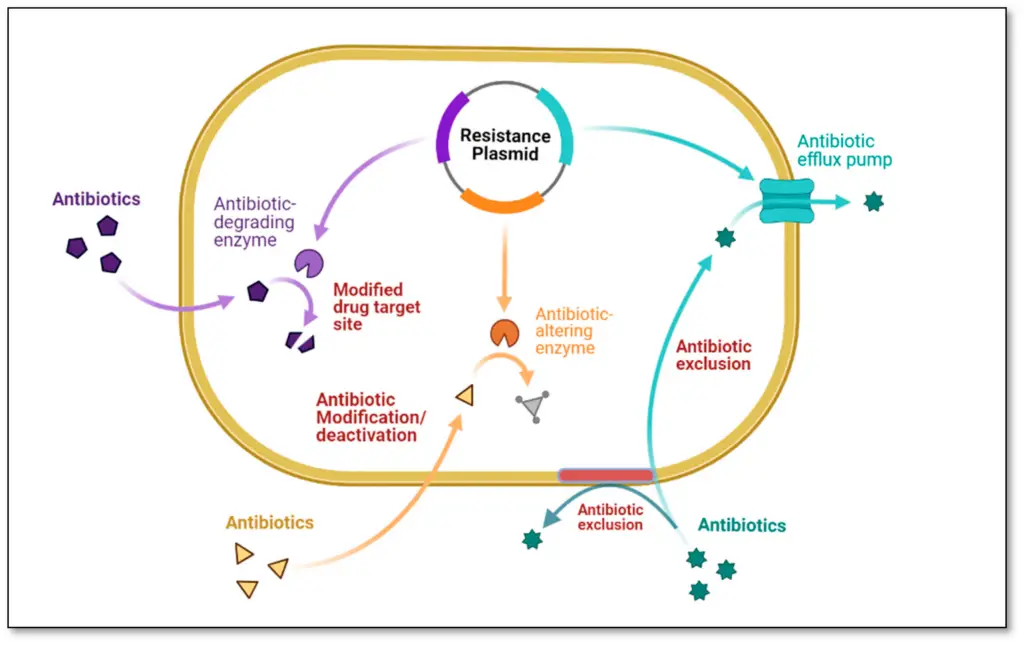
IMAGE : Mechanisms of Antibiotic Resistance in Bacteria (The image illustrates various mechanisms of antibiotic resistance in bacteria, highlighting key processes such as antibiotic exclusion, modification, and degradation. The diagram features a bacterium illustrated with a resistance plasmid, which facilitates the expression of antibiotic-altering enzymes and other factors that lead to modified drug targets. Key terms such as ‘Antibiotic-degrading enzyme,’ ‘Antibiotic efflux pump,’ and ‘Antibiotic exclusion’ are used to represent critical resistance strategies. This visual representation serves as a valuable educational tool in microbiology and pharmacology, providing insights into the molecular strategies employed by bacteria to evade antibiotic treatments.)
B. Implementation of public health policies and education programs
The implementation of comprehensive public health policies and education programs is crucial in combating the rising threat of antibiotic resistance. These initiatives aim to enhance awareness among healthcare professionals and the general public regarding the judicious use of antibiotics, thereby curbing misuse and overprescription. Strong policies must ensure equitable access to necessary antibiotics while simultaneously promoting stewardship practices that educate patients about the risks associated with inappropriate usage. Additionally, data-driven strategies, as outlined in recent reports, highlight the importance of adapting these public health frameworks to local contexts while fostering collaboration at global, regional, and national levels to mitigate AMRs spread (Bhagwandin et al.). Countries facing healthcare challenges, particularly those with a high prevalence of infectious diseases, must actively engage in tailored interventions that address unique barriers to change (Matteson et al.). The educational efforts illustrated in (Image8) emphasize the multifaceted causes of resistance, underscoring the need for systemic solutions in public health strategies.
V. Conclusion
The multifaceted challenge of antibiotic resistance necessitates a comprehensive and strategic approach to mitigate its impact on public health. Limitations and regulations on antibiotic usage, specifically in agriculture, are crucial, as the overuse of these medications has led to the emergence of resistant strains that compromise treatment efficacy in humans. This crisis underscores the urgency for innovative solutions, including alternatives such as pre-and-probiotics, vaccines, and tailored antibiotics, which may provide cost-effective therapeutic options while minimizing resistance development. Importantly, the successful management of antibiotic resistance has showcased that cycling between structurally similar antibiotics can restore susceptibility in some cases, as evidenced by recent studies (Barlow et al.). The contributions of illustrations such as elucidating bacterial resistance mechanisms can reinforce our understanding of this complex issue, emphasizing the need for intensified research and collaboration among scientists, policymakers, and healthcare professionals to foster sustainable practices in combating antibiotic resistance (Nguyen et al.).
A. Summary of key points regarding causes and solutions
The evolution of antibiotic resistance has emerged as a critical global health challenge, exacerbated by factors such as over-prescription, misuse in agriculture, and inadequate infection control measures. Understanding these causes is essential for developing effective solutions. For instance, the graphic representation of bacterial resistance mechanisms, particularly through the presence of efflux pumps and target site modifications, elucidates how rampant antibiotic use fosters resilient bacterial strains (). Addressing the issue necessitates a multifaceted approach, including improving prescription practices, implementing stricter agricultural regulations, and promoting public awareness campaigns on antibiotic usage ((Lillestolen et al.)). Moreover, enhancing surveillance systems and fostering research into novel therapeutic strategies are pivotal to curbing the spread of resistant strains ((Levidow et al.)). Ultimately, a cohesive strategy that combines education, regulation, and innovation is paramount in reversing the tide of antibiotic resistance and protecting public health.
| Cause | Example | Impact (%) |
| Overuse of Antibiotics in Healthcare | Prescribing antibiotics for viral infections | 30% |
| Agricultural Use of Antibiotics | Routine usage in livestock for growth promotion | 80% of total antibiotic use in some countries |
| Poor Infection Prevention and Control | Limited sanitation and hygiene in healthcare facilities | Increase in hospital-acquired infections |
| Patient Non-Adherence to Treatment | Not completing prescribed antibiotic courses | 50% of patients |
| Antibiotic Stewardship Programs | Promote the appropriate use of antibiotics | Up to 30% reduction in antibiotic use |
| Public Awareness Campaigns | Educate the public on antibiotic use and resistance | Increased awareness among 70% of the population |
| Research and Development of New Antibiotics | Investing in innovative treatments to combat resistance | Emergence of new drug classes in the last decade |
| Improved Infection Control Practices | Enhanced hygiene protocols in healthcare settings | Reduction of HAIs by up to 40% |
Antibiotic Resistance Overview: Causes and Solutions
B. Call to action for continued research and responsible antibiotic use
The ongoing escalation of antibiotic resistance necessitates an urgent call to action for both continued research and the promotion of responsible antibiotic use. Understanding the intricate mechanisms through which bacteria adapt and thrive despite antibiotic pressure is essential for developing effective strategies to combat this public health crisis. Research initiatives must focus not only on elucidating the biochemical pathways involved in resistance, as depicted in , but also on investigating innovative therapeutic alternatives and resistance mitigation strategies. Furthermore, public awareness campaigns promoting judicious antibiotic use in healthcare settings and agriculture must be prioritized to prevent unnecessary prescriptions that contribute to the resistance problem. By fostering collaboration among researchers, healthcare providers, and policymakers, we can create a comprehensive approach to tackling antibiotic resistance, ensuring that effective treatments remain available for future generations. Such multifaceted efforts are critical to reversing the tide of resistance and achieving long-term solutions.
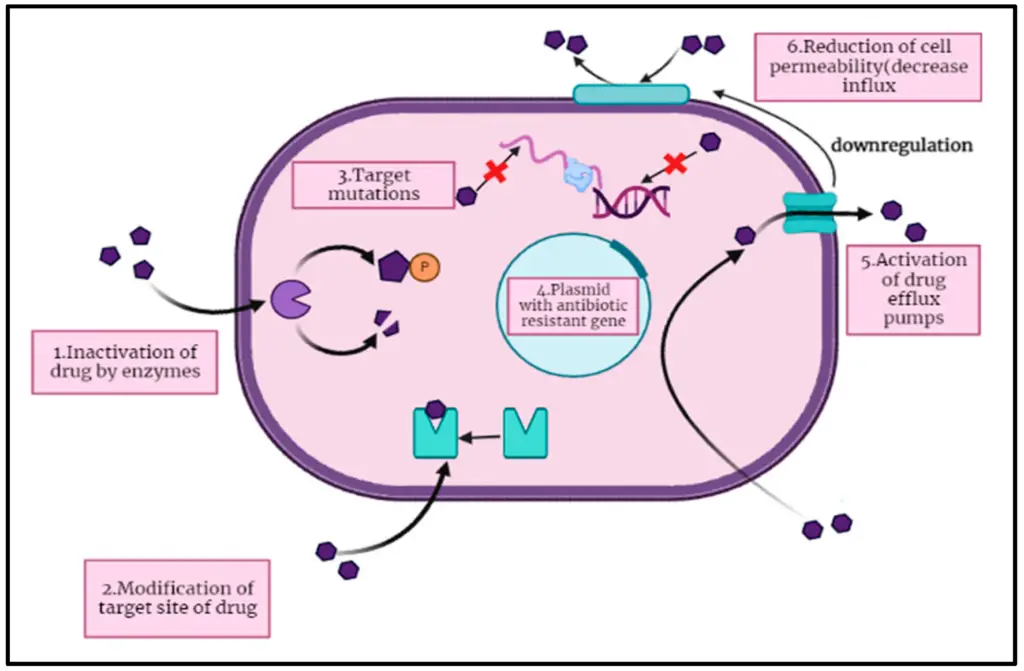
IMAGE – Mechanisms of Antibiotic Resistance in Bacteria (The diagram illustrates the mechanisms by which bacterial cells develop antibiotic resistance. It includes six distinct processes: (1) Inactivation of the drug by enzymes, (2) Modification of the drug’s target site, (3) Target mutations affecting antibiotic binding, (4) The role of a plasmid carrying antibiotic resistance genes, (5) Activation of drug efflux pumps that expel antibiotics, and (6) Reduction of cell permeability that decreases antibiotic influx. Each component is labeled and visually represented to aid in understanding the complex interactions that contribute to antibiotic resistance in bacteria.)
REFERENCES
- Birnir, Bjorn, Carpio, Ana, Cebrian, Elena, Vidal, et al.. “Dynamic energy budget approach to evaluate antibiotic effects on biofilms”. ‘Elsevier BV’, 2017, https://core.ac.uk/download/211008215.pdf
- A Armiento, A Gare, A Hilfinger, A Karmon, A Lin, A Ross-Gillespie, A Sekikawa, et al.. “Analytic philosophy for biomedical research: the imperative of applying yesterday’s timeless messages to today’s impasses”. 2020, https://core.ac.uk/download/237182315.pdf
- Akrim, Jaouad, Khayati, Youssef, Ousaid, Amine. “Overuse of antibiotics as a key driver to antibiotic resistance in Morocco: A short review with potential solutions”. ‘International Medical Publisher (Fundacion de Neurociencias)’, 2020, https://core.ac.uk/download/354489912.pdf
- Matteson, Kelsey. “Tracing the Human Healthcare Roots of Antibiotic Resistance in India: Causes, Challenges, and Promising Solutions”. SIT Digital Collections, 2015, https://core.ac.uk/download/232737642.pdf
- Lillestolen, Derek. “Infections Not Fought: Antibiotic Resistance in Underserved Communities”. Scholars Crossing, 2018, https://core.ac.uk/download/158970352.pdf
- Levidow, Les. “Precautionary Expertise for GM Crops (PEG): EU Workshop Report”. Centre for Technology Strategy, The Open University, 2003, https://core.ac.uk/download/74210748.pdf
- Barlow, Miriam, Crona, Kristina A., Goulart, Christiane P., Greene, et al.. “Designing antibiotic cycling strategies by determining and understanding local adaptive landscapes”. ‘Public Library of Science (PLoS)’, 2013, https://core.ac.uk/download/18870271.pdf
- Angelos, John A, Arens, Amanda L, Cadriel, Jessica L, Johnson, et al.. “One Health in food safety and security education: Subject matter outline for a curricular framework.”. eScholarship, University of California, 2017, https://core.ac.uk/download/323068605.pdf
- Nguyen, Ha. “Antibiotic Resistance in Livestock: A Literature Review of Its Consequences and International Effort on Human Antimicrobial Agents Resistant Crisis”. ‘Singidunum University’, 2024, https://core.ac.uk/download/624274607.pdf
- Liao, Chao, Terhune, Jeffery, Wang, Luxin, Zeng, et al.. “Impacts of florfenicol on the microbiota landscape and resistome as revealed by metagenomic analysis.”. eScholarship, University of California, 2019, https://core.ac.uk/download/323068798.pdf
- Dantas, G, Fishbein, S R S, Wallace, M J. “Antimicrobial resistance in enteric bacteria: Current state and next-generation solutions”. Digital Commons@Becker, 2020, https://core.ac.uk/download/604217549.pdf
- Bristow, Claire C, Cristillo, Anthony D, Dillon, Jo-Anne, Dong, et al.. “Antimicrobial Resistance in Neisseria gonorrhoeae: Proceedings of the STAR Sexually Transmitted Infection-Clinical Trial Group Programmatic Meeting.”. eScholarship, University of California, 2019, https://core.ac.uk/download/294625789.pdf
- Runge, Cameran. “Antimicrobial Resistance in ESKAPE Pathogens and its Effect on Modern Medicine and Treatment”. DigitalCommons@University of Nebraska – Lincoln, 2023, https://core.ac.uk/download/573442941.pdf
- Gouws, P.A., Hoffman, L.C., van den Honert, M.S.. “Importance and implications of antibiotic resistance development in livestock and wildlife farming in South Africa: A Review”. ‘African Journals Online (AJOL)’, 2018, https://core.ac.uk/download/478282323.pdf
- Carvalho, João, Cunha, Mónica V., Fernandes, Joana, Fonseca, et al.. “Mapping the scientific knowledge of antimicrobial resistance in food-producing animals”. ‘Elsevier BV’, 2021, https://core.ac.uk/download/515889753.pdf
- Bhagwandin, Niresh, Jenner, Andrew, Kowalski, Stanley P.. “Antimicrobial Resistance (AMR) and Multidrug Resistance (MDR): Overview of Current Approaches, Consortia and Intellectual Property Issues”. University of New Hampshire Scholars\u27 Repository, 2017, https://core.ac.uk/download/215505935.pdf
- De Angelis, Massimiliano, Mascellino, Maria Teresa, Oliva, Alessandra, Porowska, et al.. “Antibiotic susceptibility, heteroresistance, and updated treatment strategies in helicobacter pylori infection”. place:Macclesfield, 2017, https://core.ac.uk/download/154948401.pdf
- Todd, Kelly. “The Promising Viral Threat to Bacterial Resistance: The Uncertain Patentability of Phage Therapeutics and the Necessity of Alternative Incentives”. Duke University School of Law, 2014, https://core.ac.uk/download/213020895.pdf
- Bassegoda Puigdomenech, Arnau, Ivanova, Kristina Dimitrova, Ramon Portés, Eva, Tzanov, et al.. “Strategies to prevent the occurrence of resistance against antibiotics by using advanced materials”. ‘Springer Science and Business Media LLC’, 2018, https://core.ac.uk/download/157810784.pdf
- Miggelbrink, Judith, Molinari, Nora. “The Silenced Pandemic?: Reconstructing History and Spatiality of EU’s Biopolitics on Antimicrobial Resistance”. Leipziger Universitätsverlag, 2023, https://core.ac.uk/download/597140066.pdf
Image References:
- “Diagram of Antibiotic Resistance Gene Mobilization in Microbial Communities.” media.springernature.com, 23 January 2025, https://media.springernature.com/m685/springer-static/image/art%3A10.1038%2Fs41579-021-00649-x/MediaObjects/41579_2021_649_Fig1_HTML.png
- “Mechanisms of Antibiotic Resistance in Bacteria.” pub.mdpi-res.com, 23 January 2025, https://pub.mdpi-res.com/antibiotics/antibiotics-12-00028/article_deploy/html/images/antibiotics-12-00028-g001.png?1671879829
- “Mechanisms of Antibiotic Resistance in Bacteria.” www.mdpi.com, 23 January 2025, https://www.mdpi.com/pharmaceuticals/pharmaceuticals-16-01615/article_deploy/html/images/pharmaceuticals-16-01615-g001.png
