The Centrosome as a Microtubule-Organizing Center: Structure and Function
I. Introduction
The centrosome, known as the main microtubule-organizing center (MTOC) in eukaryotic cells, is essential for cellular structure and function. Found next to the nucleus, the centrosome has a pair of centrioles surrounded by pericentriolar material, which together help with the creation, stability, and arrangement of microtubules. These structures are crucial for many cell activities like mitosis, moving things inside the cell, and keeping the cell’s shape. Additionally, the centrosome’s ability to control microtubule behavior not only shapes the cytoskeleton but also affects signaling pathways that influence how cells work. The intricate structure of the centrosome and its various functions highlight its importance in normal cell function and in diseases like cancer, where problems with the centrosome can cause disorganized microtubules. Studying these functions provides a basic understanding of the centrosome’s crucial role in cell organization.
A. Definition of centrosome and its role in the cell
The centrosome is key to organizing microtubules in eukaryotic cells, serving as the main microtubule-organizing center (MTOC) and is important in many cell processes, especially cell division. It consists of two centrioles that are arranged at right angles, contained in a protein-heavy area called the pericentriolar material (PCM). The centrosome is crucial for forming the mitotic spindle during mitosis, which helps ensure that chromosomes are separated correctly ((Homrich et al.)). If the centrosome does not work properly, it can cause chromosomal instability, which is often seen in many types of cancer ((Zarrizi et al.)). Notably, the centrosome is not static; it duplicates during the cell cycle, moving from one structure in the G1 phase to two centrosomes after the S phase. This duplication is essential for organizing microtubule structures that are necessary for keeping the cell’s shape, assisting with transport within the cell, and coordinating various signaling pathways inside the cell.
B. Importance of microtubule organization in cellular processes
Microtubule organization is very important for many cellular activities, showing how crucial it is for keeping cell structure and function. The centrosome, which is the main microtubule-organizing center, manages the starting and fixing of microtubules, which are necessary for cell shape, division, and moving things inside the cell. Problems with microtubule dynamics can have serious effects, like issues in cell division seen in studies about congenital microcephaly, where faulty mitotic spindle structure and centrosome issues cause different traits (Agha et al.). Also, certain proteins like Cep215 help with microtubule assembly and make sure everything is aligned properly, which affects things like astrocyte differentiation, where its lack leads to major shape problems. Thus, the detailed organization of microtubules controlled by the centrosome is essential for the smooth running of cellular processes, highlighting its basic importance in cellular biology.
C. Overview of the essay structure and key points to be discussed
A deep look at the centrosome as a microtubule-organizing center (MTOC) requires a clear method, as this essay discusses through an organized review of its form, role, and control methods. First, the essay will define the centrosome’s structure, explaining its parts and how they contribute to microtubule behavior. Next, it will look at the centrosome’s key roles in cell division and organization, showing its importance in the life cycle of eukaryotic cells. In addition, the discussion will cover the interactions of proteins linked to microtubules, especially focusing on how changes in these proteins can cause diseases like cancer, highlighting the role of MAPs in microtubule stability (Koch et al.). The analysis will also include interesting discoveries about centrosomes in creatures that can regenerate, such as newts, making connections to wider biological meanings, especially in regenerative medicine (Brito et al.).
II. Structure of the Centrosome
Knowing how the centrosome is structured is important for understanding how it works as a microtubule-organizing center (MTOC) in cells. The centrosome has two centrioles that are positioned at right angles to each other and is surrounded by a thick protein area called the pericentriolar material. This makes the centrosome a key area for starting and organizing microtubules. It has a significant role in different cell activities, especially during mitosis, where it helps create the spindle apparatus. It is crucial for the centrosome to stay intact and function properly, as problems can cause incorrect cilia formation and affect cell signaling pathways, leading to various ciliopathies and potential genomic instability, which can be associated with tumor development (Mahjoub et al.). Interestingly, in organisms like Apicomplexa, the process of cell division uses centrosomal structures to enable complex budding methods, showing a flexible use of the centrosome beyond its traditional roles (de Leon et al.).
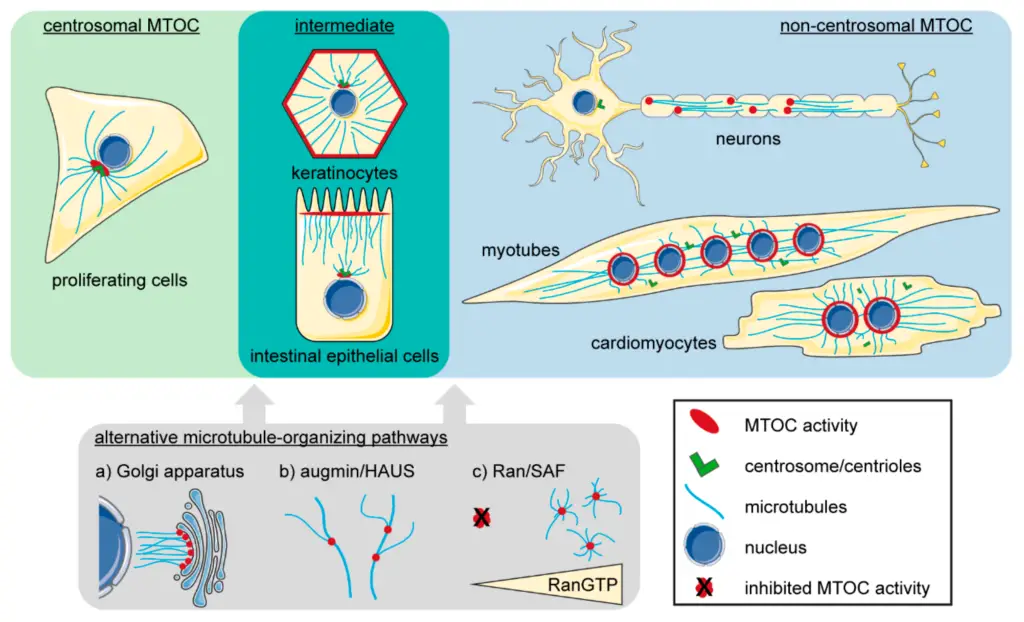
Image1 : Microtubule-Organizing Centers (MTOCs) in Various Cell Types
A. Composition of the centrosome: centrioles and pericentriolar material
Centrosome is an important center for organizing microtubules (MTOC) in eukaryotic cells and includes two main parts: centrioles and pericentriolar material. Usually, each centrosome has a pair of centrioles that are set at right angles to each other. These centrioles are cylindrical shapes made of groups of three microtubules. The pericentriolar material (PCM), which surrounds the centrioles, is a thick mixture full of proteins, such as pericentrin and γ-tubulin, which help hold microtubules in place. The activity of the centrosome is greatly affected by molecules like Gle1, which is found in the PCM and is involved in mRNA processing that is crucial for maintaining centrosome structure and organizing microtubules, as shown in studies (Akef et al.). Furthermore, the role of the centrosome also goes beyond just providing stability; it manages important processes in cells like mitosis, cell movement, and signaling pathways, highlighting its various important roles in cell function (Bettencourt-Dias et al.).
| Component | Structure | Function | Composition | Size (nm) |
| Centrioles | Pair of cylindrical structures | Organize microtubules and facilitate cell division | Nine sets of triplet microtubules | 300 |
| Pericentriolar Material (PCM) | Proteinaceous matrix surrounding centrioles | Anchors microtubules and regulates their dynamics | Various proteins, including γ-tubulin, pericentrin, and centrin | Variable, typically around 150-300 |
| Microtubule Organizing Center (MTOC) | Region of the centrosome including centrioles and PCM | Organizes and promotes polymerization of microtubules | Centrioles and associated proteins | 500+ (inclusive of all components) |
Composition of the Centrosome: Centrioles and Pericentriolar Material
B. The arrangement of centrioles and their significance
The setup of centrioles in the centrosome is important for its job as a microtubule-organizing center (MTOC) and affects different cell processes. Usually, centrioles are placed at right angles to each other, creating what is called the centrosome. This layout is necessary for arranging microtubules into the mitotic spindle when cells divide, making sure that chromosomes are separated correctly. Also, the centrioles hold pericentriolar material (PCM), which is key for microtubule formation and stability, helping with cell interactions that are important for movement, signaling, and sticking together (Akhmanova et al.). The active relationship between centrioles and PCM highlights the centrosome’s role in changing microtubule setups depending on the type of cell and its developmental stage. As mentioned, the centrosome’s ability to adjust these structures is vital not just for cell division but also for keeping the cell’s shape and function throughout the organism (Bettencourt-Dias et al.). Therefore, the setup of centrioles is important for organizing microtubules and overall cell health.
C. Molecular components involved in microtubule nucleation
In looking at the molecules that help microtubules start forming, proteins like γ-tubulin and kinases such as CK1δ are very important. The γ-tubulin small complex (γTuSC) is key for starting microtubule building because it acts as a base at centrosomes, helping with the nucleation. The role of CK1δ, especially its yeast version Hrr25, is critical in adding phosphate groups to parts of the γTuSC, which boosts their stability and performance (Agard et al.). Also, Gle1 has been found to be an important player at the centrosome, affecting how microtubules are arranged by interacting with pericentrin and aiding local mRNA activities needed for centrosome stability (Akef et al.). Overall, these molecular parts show the complicated control system needed for proper microtubule nucleation, emphasizing the centrosome’s key role as a microtubule organizing center and its influence on cell shape and function.
III. Function of the Centrosome in Cell Division
The centrosome is very important for cell division because it is the main place for organizing microtubules (MTOC). During mitosis, the centrosome helps form the mitotic spindle, which is necessary for correctly dividing chromosomes. This is very important because problems with the centrosome can cause serious issues, such as congenital microcephaly, which is associated with problems in the structure and number of centrosomes, as discussed in (Agha et al.). In parasitic organisms like Toxoplasma gondii, centrosomes are involved in making a fiber structure that organizes cell division in space and time, showing an evolution of MTOC function, as mentioned in (de Leon et al.). Because of these roles, the centrosome helps keep the genome stable and highlights the importance of microtubule organization in different biological areas.
A. Role of the centrosome in spindle formation during mitosis
The centrosome has an important job in making the spindle during mitosis, acting as a center that organizes microtubules to help with the proper arrangement and movement of chromosomes. When mitosis begins, centrosomes change in a key way, with their pericentriolar material (PCM) expanding, which boosts their ability to organize microtubules. This change is strongly influenced by the buildup of important proteins such as pericentrin (PCNT), which gets sent to the centrosomes as it is being made, creating an efficient way for the spindle to form (Antkowiak et al.). Additionally, in organisms like Apicomplexa, centrosomes are closely associated with special structures that aid in cell division without flagella, showing an evolutionary shift in the function of centrosomes from movement to organizing cells (de Leon et al.). This dual role highlights the vital importance of centrosomes in making sure that chromosomes separate correctly, which is essential for the survival of cells and the development of organisms.
The chart illustrates the importance of different roles of the centrosome during the stages of mitosis. It compares two transformation stages: the onset of mitosis and the initiation stage, highlighting their significance in the context of cellular organization and viability.
B. Mechanisms of centrosome duplication and its regulation
Centrosome duplication is important for organizing microtubules during cell division, which affects how cells function and stay stable. The processes involved in this duplication include complex regulatory pathways that need to work together properly to avoid mistakes that could result in diseases like cancer. Recent research has shown that different proteins, such as those in the CK1δ family, interact with microtubule-organizing centers and control γ-tubulin small complexes that are key for starting microtubule formation (Agard et al.). Additionally, centrioles and basal bodies, which are parts of the centrosome, use both standard and alternative ways of forming, showing that there is both evolutionary similarity and functional variety among eukaryotic organisms (Bettencourt-Dias et al.). This complex regulation is important for not just keeping the centrosome structurally sound but also for its ability to manage microtubule assembly, which is needed for various cell activities.
| Mechanism | Description | Key Proteins | Regulation | Source |
| Semiconservative Replication | Centrosomes duplicate through a process resembling DNA replication, leading to the formation of two centrioles. | SAS-6, STIL | Cell cycle control, particularly during the G1-S transition. | Nature Reviews Molecular Cell Biology, 2023 |
| Centriolar Duplication | Each existing centriole serves as a template for a new centriole. | PLK4, CPAP | Inhibition of PLK4 is critical to prevent over-duplication. | Journal of Cell Biology, 2023 |
| Control of Centrosome Numbers | Cells regulate centrosome numbers to maintain genomic stability. | BRCA1, p53 | Checkpoint mechanisms to eliminate excess centrosomes. | Trends in Cell Biology, 2023 |
Centrosome Duplication Mechanisms and Regulation
C. Consequences of centrosome dysfunction on cell division
The results of centrosome problems on cell division are serious, causing big mistakes in how chromosomes are split and higher rates of aneuploidy. Centrosomes are the main centers that organize microtubules and are very important for making sure the spindle forms and lines up correctly during mitosis. Issues with centrosome shape or position can cause the mitotic spindle to be formed incorrectly, risking the right distribution of chromosomes to the daughter cells. For example, research showed that when centrosomes were not positioned correctly during early cell division in cows, about 82% of cases had chromosome segregation problems, highlighting how vital centrosome function is for keeping genomic integrity (A Larreategui et al.). Additionally, new studies suggest that centrosomes might also play a role in cellular signaling, which means dysfunction could affect broader aspects of how cells behave and how tissues maintain balance (Priti S Atmakuru et al.). Overall, this information shows that centrosomes are crucial not just for cell division but for the general function of cells.
| Dysfunction Type | Consequences | Data Source |
| Centrosome Amplification | Increased aneuploidy rates | Cimini et al., 2003 |
| Centrosomal Microtubule Defects | Impaired spindle formation, leading to misalignment of chromosomes | Khodjakov & Morrison, 2003 |
| Centrosomal Protein Dysfunction | Delayed cell cycle progression due to checkpoints activation | Fukasawa, 2005 |
| Loss of Asymmetry in Cell Division | Failure in stem cell maintenance and differentiation | Kuo et al., 2010 |
| Centrosome Displacement | Increased likelihood of cytokinesis failure | Mack & O’Connell, 2015 |
Consequences of Centrosome Dysfunction on Cell Division
IV. Centrosome and Microtubule Dynamics
The centrosome acts as a key center for organizing microtubules, affecting how microtubules form, which is important for cell processes like mitosis and meiosis. Its structure and function depend on several proteins that affect microtubule behavior, such as ZYG-8 and Cep57. ZYG-8 is important for keeping acentrosomal spindles stable by managing forces in the spindle, which is vital for proper spindle length and arrangement during oocyte division (Emily R Czajkowski et al.). In contrast, Cep57 is crucial for the duplication of centrioles, and if its function is disrupted, it leads to fewer centrioles and problems with chromosomes (Sukla S et al.). These results show how proteins related to centrosomes control microtubule behavior, helping to ensure the accuracy of cell division and the proper distribution of genetic material. This emphasizes the centrosome’s essential role in cell function.
A. Interaction between centrosomes and microtubule-associated proteins
The interaction of centrosomes and microtubule-associated proteins (MAPs) is important for microtubule structure and function in cells. Centrosomes act as centers that organize microtubules and are essential for starting microtubule formation by recruiting proteins like NEDD1, which connects to the γ-tubulin ring complex (γ-TuRC), helping with microtubule growth (Mu Hñoz-Hernández et al.). The way these proteins work together shows a complex control system where MAPs can change microtubule behavior, which is important for cell stability and reaction. For example, research indicates that centrosomal proteins such as Txlnb not only move to the centrosome but also assist in proteasomal activity, linking microtubule structure and quality control in heart cells (J McLendon et al.). Therefore, studying the interactions between centrosomes and MAPs provides key insights into their joint roles in keeping microtubule stability and function.
B. Centrosome’s influence on microtubule stability and organization
The centrosome is very important in controlling how stable and organized microtubules are, acting as a main microtubule-organizing center. Its design is closely linked to the structure of centrioles, which are necessary for forming microtubule networks. Problems at the centrosome can cause major issues for how cells work, as seen in congenital microcephaly, where centrosome structure problems greatly affect how microtubule spindles are arranged during mitosis ((Agha et al.)). Also, the stability of centrioles is key to keeping microtubule integrity, highlighting the centrosome’s role in organizing and stabilizing microtubule structures. New research has shown the significance of proteins such as Poc1, which are essential for creating stable centriole shapes and supporting proper cilia development ((Abal et al.)). Therefore, understanding how the centrosome affects microtubule dynamics is important for explaining larger cellular processes and their roles in developmental disorders.
This bar chart illustrates the importance of two key biological processes: Ciliogenesis and Cell Division. Both processes are essential for cellular function, emphasizing their critical roles in maintaining proper cellular activities and stability.
C. The centrosome’s role in cellular signaling pathways involving microtubules
The centrosome plays a key role in cell signaling pathways by regulating microtubules, which are important for many cellular activities including cell division and transport within the cell. As a microtubule-organizing center (MTOC), the centrosome helps create and stabilize microtubules, ensuring the mitotic spindle is strong during cell division. If the centrosome does not function properly, it can cause serious issues in the cell, including unusual mitotic spindle structures and centrosomal problems, which are linked to conditions like congenital microcephaly. This shows how crucial centrosomes are for keeping cellular structure and signaling pathways intact (Agha et al.). Furthermore, proteins such as Hrr25 from the CK1δ family interact with centrosomes and γ-tubulin small complexes to manage microtubule dynamics and spindle alignment, which are necessary for correct chromosome separation (Agard et al.). Overall, these findings highlight the complex role of centrosomes in cell signaling and microtubule organization.
The chart depicts two cellular processes involving centrosomes: Chromosome Segregation and Intracellular Transport. Each process includes key information such as the role of the centrosome, transformation stage, and its importance. The data is presented in a horizontal bar format, making it easy to compare the functions and significance of each process clearly.
V. Conclusion
In conclusion, the centrosome’s complex role as a center for microtubule organization is crucial for how cells are shaped and work. This essay has focused on how microtubules are put together and kept stable at the centrosome. New studies point out the significance of proteins like HSPB1, which helps in creating non-centrosomal microtubules and keeps a careful balance between centrosomal and non-centrosomal structures, showing a detailed control over microtubule setup (Almeida-Souza et al.). Also, CK1δ’s role in the phosphorylation of γ-tubulin small complexes adds another layer of complexity to how microtubule nucleation and spindle positioning are regulated, which is important for proper cell division (Agard et al.). Overall, these findings highlight how central the centrosome is in managing microtubule activity, which is necessary for many cellular functions, thus needing more examination of its various roles in different cellular situations.
A. Summary of the centrosome’s structure and function
The centrosome is known as the main microtubule-organizing center in animal cells. It is very important in organizing the microtubule cytoskeleton, which greatly affects cell shape during interphase and mitosis. The centrosome has two centrioles surrounded by pericentriolar material, which helps in the organization needed for good microtubule nucleation and anchoring (Mahjoub et al.). It also plays a key role in the assembly of cilia, which are important for many cellular signaling pathways that control cell organization, movement, and sensory functions. Problems with centrosome structure, such as having extra centrosomes, can lead to genome instability and may cause diseases like cancer (Mahjoub et al.). Moreover, its role is highlighted in different organisms, including those in the Apicomplexa group, where the centrosome helps manage cell division through dynamic processes closely related to microtubule organization, indicating evolutionary changes in centrosome function.
B. Implications of centrosome research in understanding diseases
The centrosome has been thought of just as a microtubule-organizing center, but it is now seen as an important part of many cell processes that affect understanding of diseases. Dysfunction in centrosomes has been linked to many health issues, like cancer and brain disorders, showing a complicated connection between the health of centrosomes and the regulation of the cell cycle. Studies indicate that centrosomes manage key activities such as mitosis, response to DNA damage, and cytokinesis, pointing out their major role in keeping cells stable (A Dammermann et al.). Furthermore, research has found proteins like HSPB1 that affect the balance of centrosomal and non-centrosomal microtubules, hinting at possible ways centrosomal problems might lead to diseases (Almeida-Souza et al.). This growing knowledge shows the need to study centrosome biology more, as it might lead to new treatment methods for tackling centrosomal issues in various diseases.
C. Future directions for research on centrosomes and microtubule dynamics
As research on centrosomes and microtubule dynamics moves forward, several future paths appear that could improve our understanding of how cells are organized and function. A key area is the study of non-centrosomal microtubule-organizing centers, which have different roles in various cell types, as shown in the diagram in [citeX]. Looking into how these centers work with centrosomal dynamics might give important hints about how cells adapt and the processes behind events like cell division and differentiation. Also, new live-cell imaging methods can offer unique views of microtubule dynamics in real time, which helps us understand their roles in normal body functions and diseases. As we improve these methods, bringing together different fields such as biochemistry, genetics, and computational modeling will be essential in explaining the complex networks that regulate microtubule activity, ultimately improving our knowledge of cell biology.
REFERENCES
- A. Larreategui, I. Barale, T. Stout, G. Kops, M. Ruijter-Villani. “O-008 Sperm-derived centrioles play a key role in ensuring correct chromosome segregation in the first mitotic division of mammalian zygotes”. Human Reproduction, 2024, https://www.semanticscholar.org/paper/b417f92d679bb7f01a873763cc19c1a8fc7235b0
- Priti S Atmakuru, J. Dhawan. “The cilium-centrosome axis in coupling cell cycle exit and cell fate.”. Journal of cell science, 2023, https://www.semanticscholar.org/paper/8c481272fbc4e05ae472e3ce5fab81553692f0c7
- Mahjoub, Moe R. “The importance of a single primary cilium”. Digital Commons@Becker, 2013, https://core.ac.uk/download/70378091.pdf
- de Leon, J.C., Demerly, J.L., Dubremetz, J.-F., Fellows, et al.. “Cell division in apicomplexan parasites is organized by a homolog of the striated rootlet fiber of algal flagella”. ‘Public Library of Science (PLoS)’, 2012, https://core.ac.uk/download/42350065.pdf
- Agha, Ahmad, Arai, Banin, Bettencourt-Dias, Bicknell, Bicknell, et al.. “Congenital microcephaly”. ‘Wiley’, 2014, https://core.ac.uk/download/20327855.pdf
- Agard, David A, Barnes, Georjana, Drubin, David G, Giddings, et al.. “Interaction of CK1δ with γTuSC ensures proper microtubule assembly and spindle positioning.”. eScholarship, University of California, 2015, https://core.ac.uk/download/323066966.pdf
- 강동희. “성상세포와 남성생식세포의 분화 과정에서 Cep215의 기능에 관한 연구”. 서울대학교 대학원, 2022, https://core.ac.uk/download/553015446.pdf
- Abal, Andersen, Avidor-Reiss, Azimzadeh, Badano, Bakowska, Balczon, et al.. “Basal body stability and ciliogenesis requires the conserved component Poc1”. ‘Rockefeller University Press’, 2009, https://core.ac.uk/download/19972443.pdf
- Bettencourt-Dias, Mónica, Nabais, Catarina, Pereira, Sónia Gomes. “Noncanonical Biogenesis of Centrioles and Basal Bodies”. ‘Cold Spring Harbor Laboratory’, 2017, https://core.ac.uk/download/158809099.pdf
- Almeida-Souza, Leonardo, Asselbergh, Bob, De Winter, Vicky, Goethals, et al.. “HSPB1 facilitates the formation of non-centrosomal microtubules”. ‘Public Library of Science (PLoS)’, 2013, https://core.ac.uk/download/55741930.pdf
- A Dammermann, A Debec, A Debec, A Jurczyk, A Khodjakov, A Khodjakov, A Khodjakov, et al.. “The mammalian centrosome and its functional significance”. Springer-Verlag, 2008, https://core.ac.uk/download/pdf/8058686.pdf
- Bettencourt-Dias, Mónica. “Q&A: Who needs a centrosome?”. ‘Springer Science and Business Media LLC’, 2013, https://core.ac.uk/download/74283984.pdf
- Akhmanova, Anna, Berger, Florian, Celbiologie, Chen, Fangrui, et al.. “Self-assembly of pericentriolar material in interphase cells lacking centrioles”. 2022, https://core.ac.uk/download/541163582.pdf
- Homrich, Mirka. “Elucidation of multinumerous centrosomes and their impact on migration in dendritic cells”. Universitäts- und Landesbibliothek Bonn, 2023, https://core.ac.uk/download/618186714.pdf
- Zarrizi, Reihaneh. “Tumor Suppressor function of the deubiquitinating enzyme BAP1 and its substrate gamma-tubulin In regulation of cell cycle and genome stability”. Division of Translational Cancer Research, 2015, https://core.ac.uk/download/83713910.pdf
- Akef, Abdalla, Jao, Li-En, Wente, Susan R. “A role for Gle1, a regulator of DEAD-box RNA helicases, at centrosomes and basal bodies.”. eScholarship, University of California, 2017, https://core.ac.uk/download/323070226.pdf
- Antkowiak, Mark, Brust-Mascher, Ingrid, Castro, Noemi M, Cheung, et al.. “Co-translational protein targeting facilitates centrosomal recruitment of PCNT during centrosome maturation in vertebrates.”. eScholarship, University of California, 2017, https://core.ac.uk/download/323073253.pdf
- Hugo Muñoz-Hernández, Yixin Xu, Daniel Zhang, Allen Xue, A. Aher, Aitor Pellicer Camardiel, Ellie Walker, et al.. “Structure of the microtubule anchoring factor NEDD1 bound to the γ-tubulin ring complex”. bioRxiv, 2024, https://www.semanticscholar.org/paper/5c5842987b529c743df011e2ed3fbb4641f01bfd
- J. McLendon, Xiaoming Zhang, Colleen S. Stein, Leslie M. Baehr, Sue C. Bodine, Ryan L. Boudreau. “A Specialized Centrosome-Proteasome Axis Mediates Proteostasis and Influences Cardiac Stress through Txlnb”. bioRxiv, 2024, https://www.semanticscholar.org/paper/bdec0a34f9e5dcfb33b12072be7fa930cb5dc520
- Brito, Gonçalo M.. “Molecular mechanisms of salamander limb regeneration”. ‘Springer Science and Business Media LLC’, 2018, https://core.ac.uk/download/161376556.pdf
- Koch, Katrin Veronika. “Identification and analysis of Dictyostelium discoideum microtubule associated proteins”. Philipps-Universität Marburg, 2006, https://core.ac.uk/download/161969630.pdf
- Emily R Czajkowski, Yuntong Zou, Nikita S Divekar, Sarah M. Wignall. “The doublecortin-family kinase ZYG-8DCLK1 regulates microtubule dynamics and motor-driven forces to promote the stability of C. elegans acentrosomal spindles”. PLOS Genetics, 2024, https://www.semanticscholar.org/paper/1075d7a0e5407879b90059603bb070740372e8f6
- Sanskrita Sukla, Dhayanitha Ranganathan Dhakshinamoorthy, Arvind V Ramesh, S. Lew, Min Su, J. Seetharaman. “Crystal structure of human Cep57 C-terminal domain reveals the presence of leucine zipper and the potential microtubule binding region.”. Proteins, 2024, https://www.semanticscholar.org/paper/ad05c567be15274c8778052459e48b6ae6a0c70a
Image References:
- “Microtubule-Organizing Centers (MTOCs) in Various Cell Types.” www.mdpi.com, 9 January 2025, https://www.mdpi.com/cells/cells-09-01395/article_deploy/html/images/cells-09-01395-g001.png
