There Are 3 Types Of Bonds In DNA Double Helix Structure
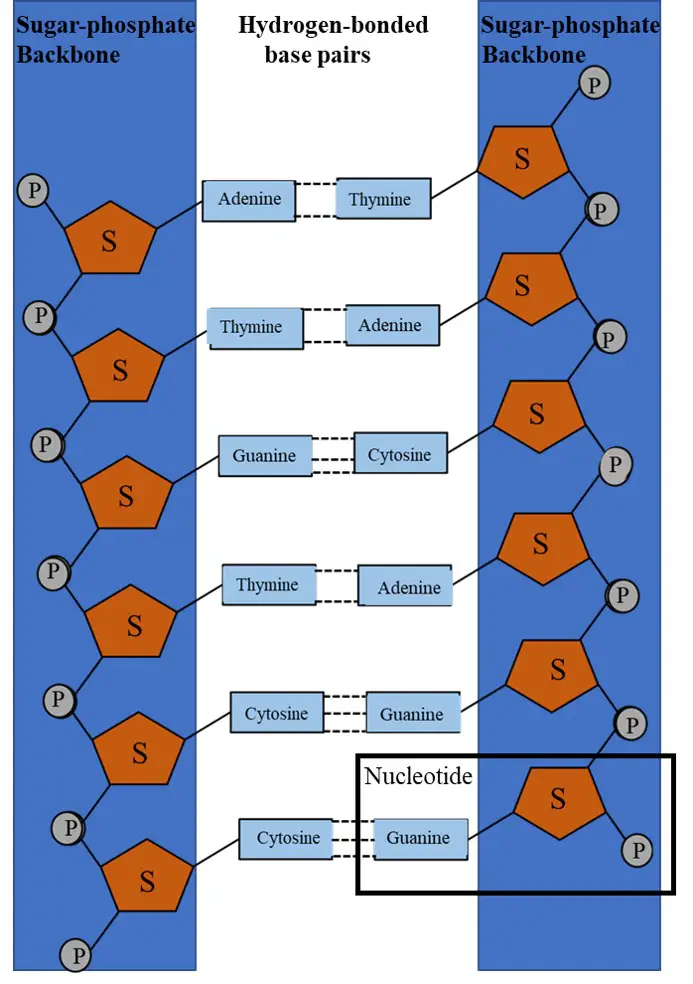
The DNA Double Helix Structure has two strands. Each strand is made up of a polynucleotide chain consisting of a Deoxyribose Pentose Sugar attached to Phosphate group with Nitrogenous bases (Adenine, Guanine, Cytosine, Thymine) being projected from the strand.
This means that in each strand of DNA you will find Deoxyribose Pentose Sugar, Phosphate Group, and Nitrogenous Bases.
One stand is attached to the other strand forming a twisted ladder-like helical structure because of the complementary base pairing between nitrogenous bases of the two strands.
There Are 3 Types Of Bonds In DNA Double Helix Structure
| NO. | BOND TYPE | BOND NATURE | BOND DETAIL |
|---|---|---|---|
| 1. | Glycosidic Bond | Strong Covalent Bond | Formed between a Nitrogenous Base and the Deoxyribose Pentose Sugar. |
| 2. | Phospho-diester Bond | Strong Covalent Bond | Formed by Phosphate Group between two Deoxyribose Pentose Sugar connecting each other to form the backbone of the DNA. |
| 3. | Hydrogen Bond | Weak Dipole-Dipole Interacting Bond | Formed between the Nitrogenous Bases of the two strands of DNA. |
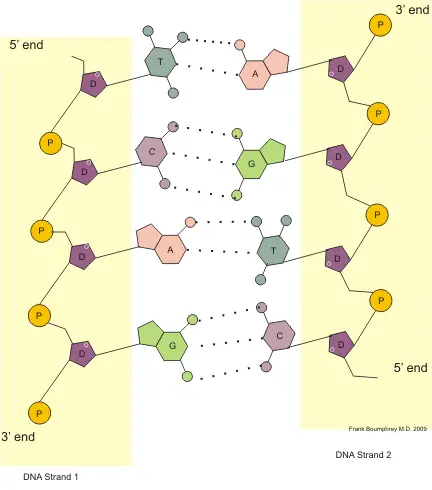
DNA & Its Bonds: Explanation of the above Diagram
In the above picture, the two strands of DNA are shown. One strand is from 5′ to 3′ (DNA Strand 1) and the other strand is from 3′ to 5′ (DNA Strand 2).
In each strand:
- D= Deoxy-ribose pentose sugar,
- P= Phosphate group,
- A= Adenine, G= Guanine, C= Cytosine, T= Thymine.
The formed bonds:
- The bond formed between D→P→D is Phospho-diester Bond.
- The bond formed between D→A, D→G, D→C, D→T is Glycosidic Bond.
- The bond formed between A→T, G→C, T→A, C→G is Hydrogen Bond.
1. Glycosidic Bond: A Covalent Bond Between a Nitrogenous Base and Deoxyribose Pentose Sugar
A glycosidic bond is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
Here, in the case of DNA, the glycosidic bond is formed between the Deoxyribose Pentose Sugar of the DNA strand with a Nitrogenous base (either Adenine, Guanine, Cytosine, or Thymine).
This glycosidic bond is also known as the N-glycosidic bond because the anomeric carbon of the sugar forms the bond with the nitrogen atom of an amine of the base.
In DNA, glycosidic bond is the nitrogen-carbon linkage between the 9′ nitrogen of purine bases (Adenine/Guanine) or 1′ nitrogen of pyrimidine bases (Cytosine/Thymine) and the 1′ carbon of the deoxyribose sugar group.
The linking of the nitrogenous base to deoxyribose sugar through N-glycosidic linkage results in the formation of nucleoside.
The N-glycosidic bond, that is especially formed by purine bases, is one of the most reactive covalent bonds in nucleic acids. N-glycosidic bonds in ribonucleosides of nucleic acids are more stable than in deoxyribonucleosides.
Nevertheless, the purine N-glycosidic bonds are more easily cleaved than pyrimidine ones.
N-Glycosid bond causes a condensation reaction, meaning that water is released. The releasing water includes the Hydroxyl (OH) group of the 1’C deoxyribose pentose sugar and the Hydrogen associated with the 9’N atom of the purine or the 1’N atom of the pyrimidine.
2. Phospho-diester Bond: A Covalent Bond Between two Deoxyribose Pentose Sugar
Phospho-diester bond occurs when exactly two of the hydroxyl groups in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds.
In the case of DNA, the phospho-diester bond is a covalent bond in which a phosphate group (PO₄³⁻) joins adjacent carbons of the deoxyribose pentose sugar through ester linkages.
This bond is the result of a condensation reaction between a hydroxyl (OH) group of two sugar groups and a phosphate group.
This bond links two nucleotides together to form oligonucleotide polymers. It links a 3′ carbon to a 5′ carbon in a DNA strand.
As the Phospho-diester bonding starts to take place inorganic Phosphoric Acid (H3PO4) is changed to Phosphate group (PO₄³⁻) where one ester bond is formed at one (O–) end of the Phosphate group and the other ester bond is formed at the other (O–) end of the Phosphate group.
Its called Phospho-diester bond because two nos. of Phospho-easter bond is created by one single phosphate group in order to join two pentose sugars together.
A corresponding nucleotide is formed by the attachment of a phosphate group to the 5’C—OH of a nucleoside through phospho-easter linkage.
A dinucleotide is formed by the linkage of two nucleotides through the 5’C & 3’C Phospho-diester linkage.
And consequently a polynucleotide is formed by the linking of many nucleotides.
3. Hydrogen Bond: Dipole-Dipole Interacting Bond Between two Nitrogenous Bases of the two strands of DNA
Hydrogen Bond is a type of non-covalent bond between complementary base pairs of the DNA helix structure that shows Dipole-Dipole interactions.
These bonds are very weak and non-covalent bonds, but provide great stability to the structure.
It occurs between any two complementary nitrogenous bases of the two different DNA strands.
Hydrogen Bonding occurs complementary meaning that Adenine bonds with Thymine by two hydrogen bonds, Cytosine bonds with Guanine by three hydrogen bonds, Thymine with Adenine by two hydrogen bonds, and Guanine with Cytosine by three hydrogen bonds.
The hydrogen bonding occurs between the electronegative O and N atoms with free lone pairs of the two complementary bases. The electronegative O and N atoms are the potential hydrogen bond acceptors.
The electronegative O and N atoms create bonds with Hydrogen atoms of the complementary base. The H atoms have a strong partial positive charge and are potential hydrogen bond donors.
A purine (Adenine or Guanine) will form hydrogen bonding with complementary pyrimidine (Cytosine and Thymine) based on the electronegative O, N interaction with the electropositive H.
So, that’s why Guanine and Cytosine make up a nitrogenous base-pair because their available hydrogen bond donors and hydrogen bond acceptors pair with each other in space.
And also that’s why Adenine and Thymine similarly pair via. hydrogen bond donors and acceptors; however an A-T/T-A base pair has only two hydrogen bonds between the bases.
What type of bond holds DNA together?
There are two major types of bond with its sub-types that holds the DNA together:
- Covalent Bonds – It occur within each linear strand and strongly bond the nitrogenous bases, deoxyribose phosphate sugars, and phosphate groups together creating each DNA stand.
- Glycosidic Bonds: It is the Nitrogen-Carbon linkage between the 9′ Nitrogen of purine bases or 1′ Nitrogen of pyrimidine bases and the 1′ Carbon of the sugar group.
- Phospho-diester bonds: It occur between the phosphate group and two deoxyribose pentose sugar molecules by a 3′ carbon to a 5′ carbon in DNA.
- Hydrogen Bonds – It occurs between the two strands and involve a nitrogenous base from one strand with a nitrogenous base from the second in complementary pairing.
- Double Hydrogen Bonds: It occur between Adenine and Thymine. It’s because the H, N of Adenine reacts with O, H of Thymine forming two hydrogen bonds.
- Triple Hydrogen Bonds: It occur between Guanine and Cytosine. It’s because the O, H, H of Guanine reacts with H, N, O of cytosine forming three hydrogen bonds.
Why are hydrogen bonds weak that the covalent bonds?
The covalent bond is a primary chemical bond formed by the sharing of electron pairs.
Covalent bonds are strong bonds with greater bond energy. It is formed due to the mutual sharing of electrons between atoms.
In DNA, the covalent bond energy in the Phospho-diester and the N-Glycosidic bond is comprised of more than 100kJ/mol.
The hydrogen bond is a weak electrostatic attraction between the hydrogen and an electronegative atom due to their difference in electronegativity.
It is formed just due to the electrostatic attraction with the hydrogen and an electronegative atom.
In DNA, the hydrogen bonds are present between the nitrogenous bases with a smaller bond energy of something 21 kJ/mol.
Therefore, it is clear that hydrogen bonds are weak than the covalent bonds. However in DNA, individual hydrogen bonds are weak bonds, however, their presence in large number provide them considerable strength and thus it gives a bit of stability and balance to the helical structure.
It is also very important to note that the structure of the DNA is very stable because of the presence of strong covalent bonds. This also indicates why DNA is stable up to a temperature of about 76.2°C. Above 76.2°C, breakdown of DNA occurs leading to a decrease in entropy as indicated by absorption of heat.
However, the Hydrogen Bonds between the complementary base pairs are aren’t very stable and strong. This helps the DNA strands to separate in a minimum requirement of ATP energy during the replication process. This one is a big advantage of hydrogen bonding in the DNA.
Nitrogenous Bases in DNA & RNA
Nitrogenous Bases are of two types:
- Purine: Contains two heterocyclic ring. Eg. Adenine, Guanine.
- Pyrimidine: Contains one heterocyclic ring. Eg. Cytosine, Thymine, Uracil.
Nitrogenous bases in DNA (Deoxyribonucleic acid):
- Adenine (A): (C5H5N5) 6-aminopurine
- Guanine (G): (C5H5N5O) 2-amino-6-hydroxypurine
- Cytosine (C): (C4H5N3O) 4-aminopyrimidin-2(1H)-one
- Thymine (T): (C5H6N2O2) 5-methyluracil
Nitrogenous bases in RNA (Ribonucleic acid):
- Adenine (A): (C5H5N5) 6-aminopurine
- Guanine (G): (C5H5N5O) 2-amino-6-hydroxypurine
- Cytosine (C): (C4H5N3O) 4-aminopyrimidin-2(1H)-one
- Uracil (U): (C4H4N2O2) 2,4-dihydroxypyrimidine
In DNA:
- Adenine is bonded to Thymine by 2 — Hydrogen bonds.
- Guanine is bonded to Cytosine by 3– Hydrogen bonds.
In RNA:
- Adenine is bonded to Uracil by 2 — Hydrogen bonds.
- Guanine is bonded to Cytosine by 3 — Hydrogen bonds.
