Are DNA and RNA Polymers? Know the Structure of Polymers
ANSWERED: Are DNA and RNA Polymers?
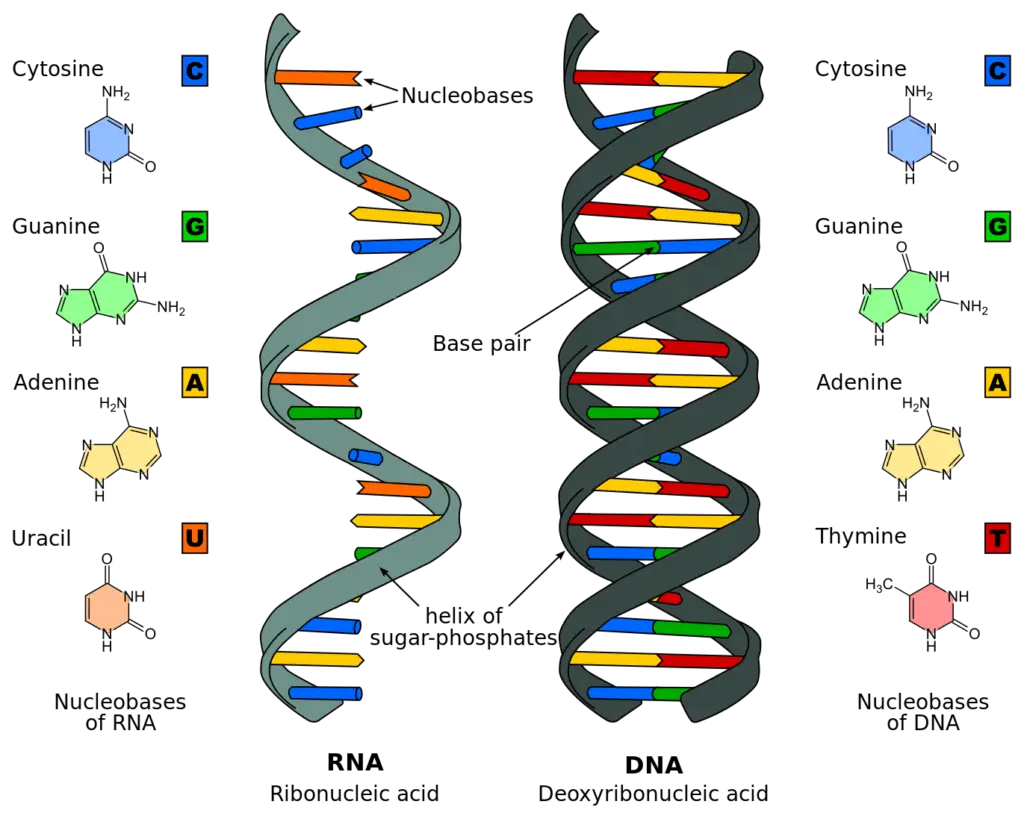
Both DNA and RNA are made up of nucleotides. Each nucleotide is composed of a nitrogenous base, a five-carbon pentose sugar, and a phosphate group.
Yes, DNA and RNA are polymers of nucleotides. In both DNA & RNA, the monomers are known as nucleotides. All of the nucleotides combine together to form a polynucleotide. The polynucleotide is the polymer of many nucleotides.
So, How DNA and RNA are polymers? Let’s understand the concept in a very simple form…
A Monomer is a single molecule that acts as a single unit. A polymer is formed when many of such monomers join together.
Here, in the case of DNA & RNA, the monomers are the nucleotides and the polymers are the polynucleotides.
Monomeric nucleotides are the repeating units throughout the entire length of the DNA/RNA molecule inside each cell.
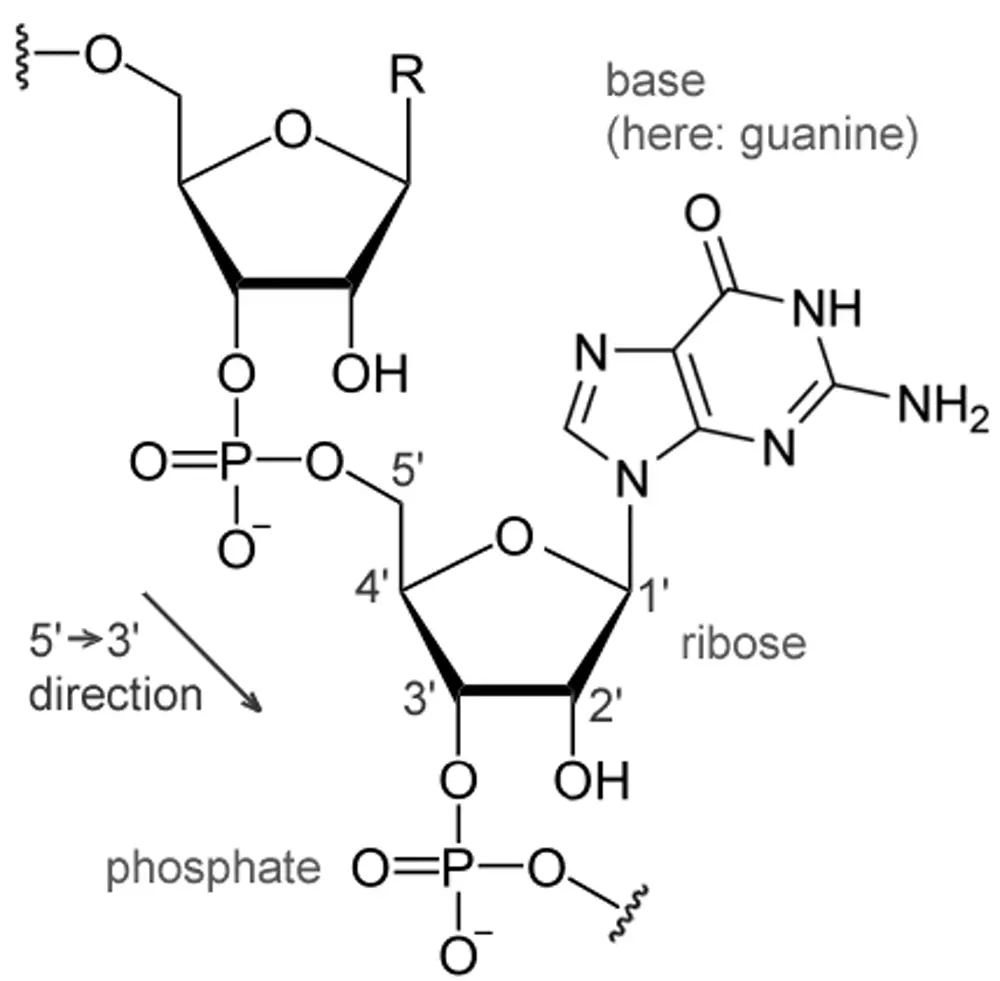
Each nucleotide is formed when a nitrogenous base (A/G/C/T/U) is attached to a pentose sugar molecule through an N-glycosidic bond, which is attached to one or more phosphate groups via. Phospho-diester Bond.
In DNA, each nitrogenous base (A/G/C/T) in a nucleotide is attached to a Deoxyribose Pentose sugar molecule, which is attached to one or more phosphate groups.
And in RNA, each nitrogenous base (A/G/C/U) in a nucleotide is attached to a Ribose Pentose sugar molecule, which is attached to one or more phosphate groups.
Both contain nucleotide as a monomer and the only difference is that its Deoxyribose Sugar and bases A/G/C/T in DNA. And, its Ribose Sugar and bases A/G/C/U in the case of RNA.
[Image Source: Wikipedia]
What’s in the Polymer of DNA
DNA is a polymer of nucleotides. Each nucleotide is formed of:
- Deoxyribose Pentose Sugar
- Phosphate Group
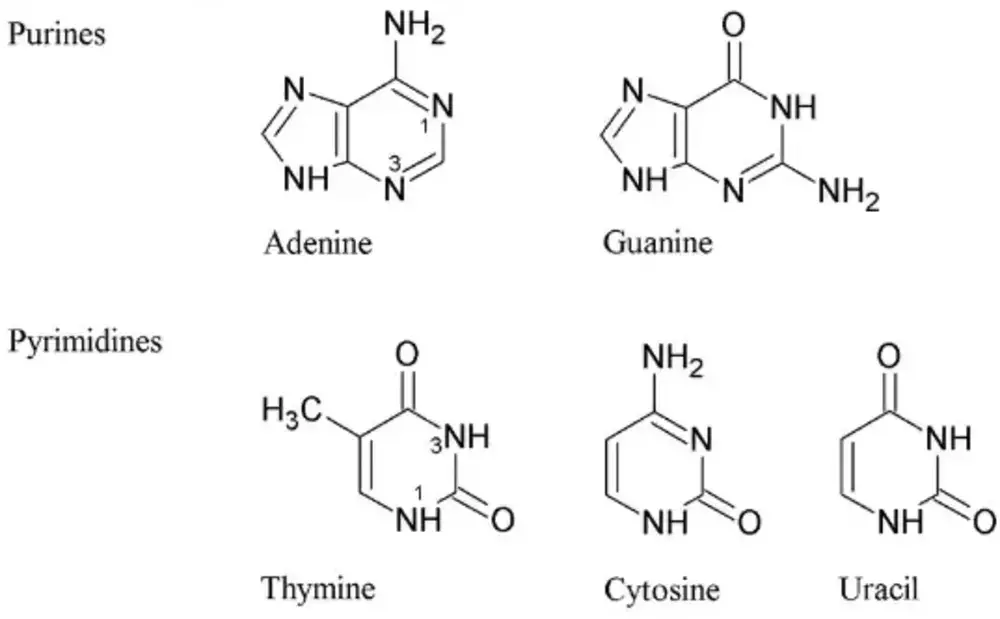
- One of any four Nitrogenous bases: Adenine(A), Guanine(G), Cytosine(C), and Thymine(T)
A polymer of DNA is one huge DNA molecule that can contain various nucleotides. In fact, each polymer can contain millions of nucleotides.
We all know chromosomes, right? These are the genetic information carriers during cell division.
Chromosomes are actually made up of two DNA polymers that stick together via. non-covalent hydrogen bonds between the nitrogenous bases of each nucleotide.
In doing so, chromosomal DNA actually consists of two DNA polymers that make up a 3-dimensional (3D) structure called a double helix.
DNA polymer is more stable than RNA polymer. DNA being a double-stranded molecule along with very strong hydrogen bonds between the nucleotide of each strand makes it very stable.
What’s in the Polymer of RNA
RNA is also a polymer of nucleotides. Each RNA nucleotide is formed of:
- Ribose Pentose Sugar
- Phosphate Group
- One of any four Nitrogenous bases: Adenine(A), Guanine(G), Cytosine(C), and Uracil(U)
RNA has Uracil instead of Thymine. The other three N-bases viz. Adenine, Guanine, and Cytosine are the same as in the case of DNA.
Uracil (U) base is also a pyrimidine and is very similar to that of DNA’s thymine (T).
Uracil is energetically less expensive to produce than thymine, which may account for its use in RNA. That’s evidence of evolution in the molecular biology of RNA and shows how RNA emerged first, before DNA.
RNA is actually single stranded in nature. The are three kinds of RNA polymer: messenger RNA (m-RNA), Ribosomal RNA (r-RNA), and transfer RNA (tRNA).
RNA polymer is less stable than DNA polymer. Mainly because it’s single-stranded and also due to the presence of (OH) hydroxyl group on the 2′ position of Pentose sugar makes RNA less stable than DNA as it becomes more susceptible to hydrolysis.
Structure of DNA polymer
We have previously learned that the DNA polymer is a polynucleotide. This means that many nucleotides act as monomers to form the DNA polymer.
Each such nucleotide is made up of Deoxyribose Pentose Sugar, Phosphate Group, Nitrogenous bases.
In a DNA polymer, there needs to be some kind of biochemical bond to hold each of the structures in order to create the polymer. These bonds are Glycosidic Bonds, Phospho-diester bonds, and Hydrogen Bonds.
Let’s know about the structure of the whole DNA polymer and the bonds that hold each monomer of the polymer together.
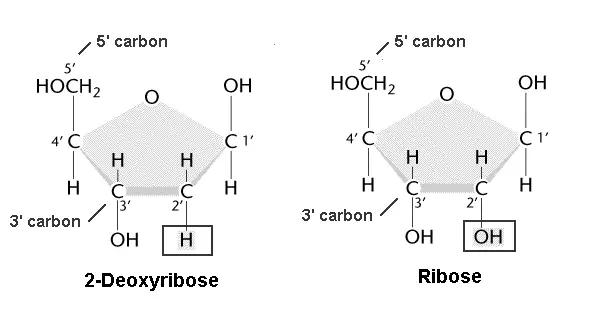
1. Deoxyribose Pentose Sugar
Carbon residues in the pentose are numbered 1′ through 5′. The nitrogenous base is attached to the 1′ position of the pentose sugar, and the phosphate is attached to the 5′ position.
It’s due to the 5 carbon similar structure that its called are called pentose sugar.
And also, it’s due to the absence of OH and presence of H in the 2′ position that it’s called Deoxyribose pentose sugar in DNA.
2. Phosphate Group
The phosphate group is a molecule containing one atom of phosphorus covalently bound to four oxygen atoms, one of which is expressed as hydroxyl (OH) group.
They are relatively reactive molecules that readily form phosphodiester bonds.
3. Nitrogenous Bases
In DNA, four types of nitrogenous bases are found:
- Adenine (A): (C5H5N5) 6-aminopurine
- Guanine (G): (C5H5N5O) 2-amino-6-hydroxypurine
- Cytosine (C): (C4H5N3O) 4-aminopyrimidin-2(1H)-one
- Thymine (T): (C5H6N2O2) 5-methyluracil
4. Biochemical Bonds
In a DNA polymer, the various biochemical bonds that are formed in a nucleotide and in joining two or more nucleotides together are provided below. These are:
- Glycosidic Bonds: It is the Nitrogen-Carbon linkage between the 9′ Nitrogen of purine bases or 1′ Nitrogen of pyrimidine bases and the 1′ Carbon of the sugar group.
- Phospho-diester bonds: It occurs between the phosphate group and two deoxyribose pentose sugar molecules by a 3′ carbon to a 5′ carbon in DNA.
- Hydrogen Bonds: It occurs between Adenine and Thymine via. two hydrogen bonds, and between Guanine and Cytosine via. three hydrogen bonds. Such bonding occurs joining the two strands of DNA.
Structure of RNA polymer
We know that the RNA polymer is a polynucleotide. This means that many nucleotides act as monomers to form the RNA polymer.
Each such nucleotide is made up of Ribose Pentose Sugar, Phosphate Group, Nitrogenous bases.
In an RNA polymer, there needs to be some kind of biochemical bond to hold each of the structures in order to create the polymer. These bonds are Glycosidic Bonds, Phospho-diester bonds, and Hydrogen Bonds.
Let’s know about the structure of the whole RNA polymer and the bonds that hold each monomer of the polymer together.
1. Ribose Pentose Sugar
Carbon residues in the ribose pentose sugar are numbered 1′ through 5′. The nitrogenous base is attached to the 1′ position of the pentose sugar, and the phosphate is attached to the 5′ position.
It’s due to the 5 carbon similar structure that its called are called pentose sugar.
And also, it’s due to the presence of OH in the 2′ position that it’s called Ribose pentose sugar in RNA.
2. Phosphate Group
The phosphate group is basically the same in both DNA & RNA. There’s no difference at all.
The phosphate group is a molecule containing one atom of phosphorus covalently bound to four oxygen atoms. Its chemical formula is PO₄³⁻.
3. Nitrogenous Bases
In RNA, four types of nitrogenous bases are found:
- Adenine (A): (C5H5N5) 6-aminopurine
- Guanine (G): (C5H5N5O) 2-amino-6-hydroxypurine
- Cytosine (C): (C4H5N3O) 4-aminopyrimidin-2(1H)-one
- Uracil (U): (C4H4N2O2) 2,4-dihydroxypyrimidine
4. Biochemical Bonds
In an RNA polymer, the various biochemical bonds that are formed in a nucleotide and in joining two or more nucleotides together are provided below. These are:
- Glycosidic Bonds: It is the Nitrogen-Carbon linkage between the 9′ Nitrogen of purine bases or 1′ Nitrogen of pyrimidine bases and the 1′ Carbon of the sugar group.
- Phospho-diester bonds: It occurs between the phosphate group and two Ribose pentose sugar molecules by a 3′ carbon to a 5′ carbon in RNA.
- Hydrogen Bonds: It occurs between Adenine and Uracil via. two hydrogen bonds, and between Guanine and Cytosine via. three hydrogen bonds. Such bonding joins the same single strand of RNA at its various points creating RNA loops.

