Mitochondria and Evolution: The Endosymbiotic Theory
I. Introduction
Within the realm of cellular evolution, one of the most revolutionary concepts is the endosymbiotic theory, which posits that mitochondria originated from free-living bacteria that entered into a symbiotic relationship with ancestral eukaryotic cells. This relationship is underscored by notable similarities between mitochondria and certain prokaryotes, particularly in their double-membrane structures and the presence of circular DNA, reminiscent of bacterial genomes. Such parallels suggest a shared lineage and bolster the argument that mitochondria were once independent organisms. Over time, this symbiosis has transitioned into a profound mutual dependence; mitochondria not only contribute to the host cells energy metabolism through aerobic respiration but also have become indispensable for cellular functions. Thus, the evolutionary journey of mitochondria exemplifies the intricate connections that underpin the diversity of life, highlighting how symbiotic partnerships have shaped cellular complexity and functionality.
A. Definition of mitochondria and their role in eukaryotic cells
The evolutionary trajectory of mitochondria illustrates a profound relationship between eukaryotic cells and their ancient prokaryotic ancestors, fundamentally reshaping our understanding of cellular function and organization. These organelles, characterized by double membranes, not only resemble the structure of contemporary bacteria but also contain their own circular DNA, akin to that found in prokaryotes. This genetic autonomy hints at their origin from an endosymbiotic event, where an ancestral eukaryotic cell engulfed an aerobic bacterium, leading to a mutually beneficial relationship. Over time, this interaction fostered a level of dependency; mitochondria became indispensable for ATP production through oxidative phosphorylation, while eukaryotic cells provided a protected environment and essential nutrients for their prokaryotic counterparts. This evolving partnership exemplifies the complexity and interdependence foundational to eukaryotic life, reinforcing the significance of mitochondria not merely as energy producers but as integral components of cellular identity and function (Militello et al.).
B. Overview of the endosymbiotic theory
The intricate relationship between mitochondria and their prokaryotic ancestors reveals a compelling narrative of evolutionary innovation. Both mitochondria and bacteria exhibit striking similarities, such as their double membranes, which provide a crucial basis for the endosymbiotic theory. This theory posits that ancient eukaryotic cells engulfed aerobic bacteria, leading to a symbiotic relationship that gradually evolved over millions of years. Notably, mitochondria possess their own circular DNA, akin to that of bacteria, reinforcing evidence of their prokaryotic origins (Leister et al.). This relationship transitioned from one of mere coexistence to mutual dependence, as the host cell provided a stable environment while mitochondrial ancestors enhanced energy production through aerobic respiration. As such, the evolutionary journey highlights how initial interactions between disparate life forms can result in complex cellular architecture vital for the development of eukaryotic organisms, fundamentally altering the trajectory of life on Earth.
C. Importance of understanding the evolutionary origin of mitochondria
A thorough investigation into the evolutionary origin of mitochondria is critical to understanding cellular complexity and the emergence of eukaryotic life. Central to this inquiry is the endosymbiotic theory, which posits that ancestral eukaryotic cells incorporated free-living bacteria, ultimately leading to the development of mitochondria. This relationship is evident in the structural and functional similarities between mitochondria and contemporary prokaryotes; both possess double membranes, their own circular DNA, and ribosomes akin to those found in bacteria. Such similarities suggest a shared evolutionary past, supporting the notion that mitochondria were once autonomous entities that evolved into obligate symbionts, resulting in a state of mutual dependence that is vital for energy metabolism in modern eukaryotes. This understanding not only enhances our grasp of evolutionary biology but also emphasizes the intricate ecological relationships that have shaped the trajectory of life on Earth (Beckley et al.).
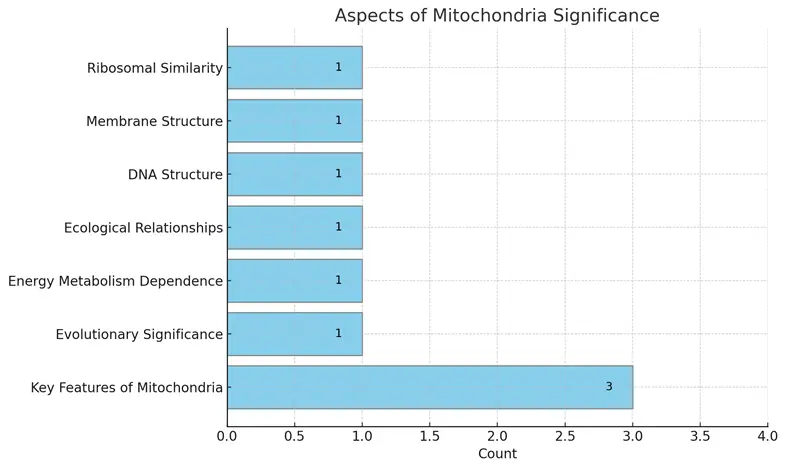
The bar chart displays the significance of various aspects of mitochondria, highlighting the most relevant features such as their structural similarities with prokaryotes and their importance in energy metabolism. Each aspect is represented along the vertical axis, while the horizontal axis indicates the count of references for each feature. This visualization allows for easy comparison of the different facets of mitochondrial significance.
II. Historical Context of the Endosymbiotic Theory
The intricate symbiotic relationship between early eukaryotic cells and engulfed prokaryotes illustrates a pivotal evolution in cellular complexity, foundational to the endosymbiotic theory. Mitochondria, often referred to as the powerhouse of the cell, share remarkable similarities with bacteria, including their double-membrane structure and circular DNA, which is reminiscent of prokaryotic genomes. These shared characteristics not only support the hypothesis that mitochondria once existed as independent aerobic bacteria but also highlight the evolutionary transition to mutual dependence between host cells and their engulfed counterparts. As the host eukaryotic cells increasingly relied on mitochondria for efficient energy production, these organelles in turn became integral components of cellular metabolism, illustrating a dynamic shift from autonomy to interdependence. This evolutionary narrative exemplifies the profound ecological and evolutionary forces shaping cellular life, validating the endosymbiotic theory as an essential framework for understanding the origins of complex organisms (Szathmáry et al.), (Archibald et al.).
| Year | Event | Source |
| 1883 | German zoologist Heinrich Anton de Bary proposes symbiosis playing a role in evolution. | De Bary, H. A. (1883). ‘The nature and causes of the manipulation of organic beings.’ |
| 1966 | Lynn Margulis publishes a paper proposing the Endosymbiotic Theory, suggesting that mitochondria originated from free-living prokaryotes. | Margulis, L. (1966). ‘On the Origin of Mitosing Cells.’ |
| 1970 | Margulis elaborates on her theory in ‘Origin of Eukaryotic Cells’, providing substantial morphological and genetic evidence. | Margulis, L. (1970). ‘Origin of Eukaryotic Cells.’ |
| 1996 | Mitochondrial DNA sequencing strengthens the endosymbiotic theory by showing similarities with alpha-proteobacteria. | Andersson, S. G. E., & Kurland, C. G. (1996). ‘The origins of mitochondria and chloroplasts.’ |
| 2013 | Genomic analysis of various eukaryotic organisms revealed more about the evolutionary history of mitochondria. | Williams, B. A., et al. (2013). ‘The genetic evolution of the jellyfish.’ |
Key Milestones in the Development of the Endosymbiotic Theory
A. Early observations and hypotheses regarding mitochondria
The intricate relationship between eukaryotic cells and mitochondria showcases a fascinating evolutionary journey, rooted deeply in ancient symbiotic interactions. Initial observations revealed significant morphological similarities between mitochondria and prokaryotes, particularly their double membranes and distinct circular DNA, which resemble that of bacteria. These similarities were pivotal in formulating the endosymbiotic theory, positing that ancestral eukaryotic cells engulfed aerobic bacteria that eventually transitioned into mitochondria, transforming cellular respiration and energy metabolism (cite10). Over time, this symbiotic relationship evolved into a state of mutual dependence, where eukaryotic hosts relied on mitochondria for efficient ATP production, while mitochondria benefited from a stable environment and nutrient access. The co-evolution of these two once-independent entities underscores their integral roles in the complexity of eukaryotic life, revealing a compelling narrative of adaptation and interdependence in the tree of life. Such dynamics exemplify the transformative power of endosymbiosis in evolutionary biology.
B. Key figures in the development of the endosymbiotic theory
The evolutionary narrative of mitochondria is steeped in the pioneering work of key figures who championed endosymbiotic theory, such as Lynn Margulis. By meticulously studying the morphological and genetic similarities between mitochondria and certain bacteria, Margulis provided compelling evidence for the idea that these organelles descended from aerobic prokaryotes. Mitochondria possess unique characteristics akin to their bacterial ancestors, including a double-membrane structure and circular DNA, which closely resembles bacterial genomes. This genetic independence not only underscores their prokaryotic origins but also suggests a once self-sufficient lifestyle prior to engulfment. Over time, a profound mutual dependence evolved; while host cells gained enhanced energy production capabilities through the oxidative processes facilitated by mitochondria, the latter became integral to cellular function and energy metabolism. Such interdependence illustrates a critical evolutionary partnership, pivoting the trajectory of eukaryotic development and reshaping our understanding of cellular life (Katz et al.), (Szathmáry et al.).
C. Evolution of scientific acceptance of the theory
The intricate relationship between mitochondria and their prokaryotic ancestors has shifted scientific paradigms over time, illustrating a profound mutual dependence that underscores the endosymbiotic theory. Early resistance to this concept stemmed from a lack of empirical evidence linking eukaryotic organelles to bacterial origins. However, advances in molecular genetics and comparative genomics have revealed striking similarities between mitochondria and bacteria, notably their double membranes and the presence of circular DNA akin to bacterial genomes. Such parallels have reinforced the notion that mitochondria evolved from free-living α-proteobacteria, integrating into a primordial eukaryotic cell through phagocytosis. As research progressed, the theory gained widespread acceptance, reflecting a broader understanding of evolutionary biology, including the roles of mitochondrial dysfunction in diseases like cancer, where mutations can lead to metabolic alterations that foster pathological states (Jianping et al.). This evolution of scientific thought exemplifies the dynamic interplay between discovery and understanding in the field of biology (Archibald et al.).
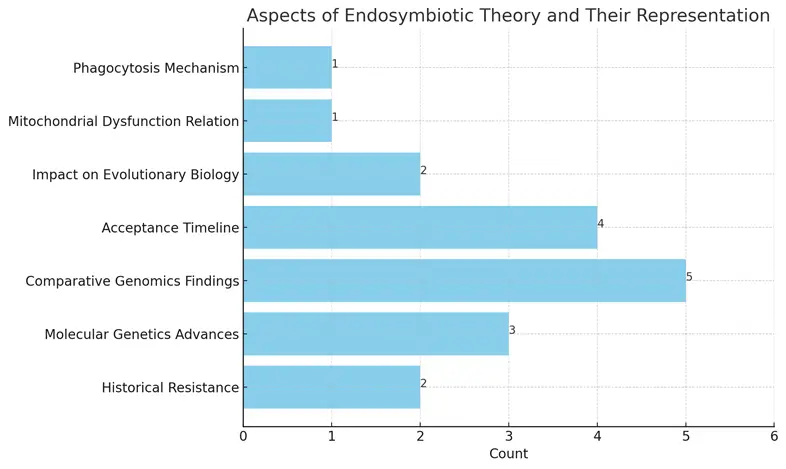
The chart displays various aspects of the endosymbiotic theory along with their representation counts. Each aspect is represented as a horizontal bar, indicating the number of supporting findings or influences associated with it. The comparative genomics findings show the highest count, suggesting significant evidence for the mitochondrial origins, while both mitochondrial dysfunction relation and phagocytosis mechanism have the lowest counts, indicating lesser emphasis in the discussion of the theory.
III. Structural and Functional Similarities Between Mitochondria and Bacteria
The intricate relationship between mitochondria and bacteria illustrates a profound evolutionary narrative rooted in endosymbiosis, demonstrating remarkable structural and functional similarities. Mitochondria possess a double membrane, mirroring the cellular membranes found in prokaryotic organisms, further substantiated by the presence of their own circular DNA, akin to bacterial genomes. This shared characteristic suggests a lineage derived from free-living bacteria, likely α-proteobacteria, that once operated independently before becoming integral to eukaryotic cells. Over time, an evolutionary path of co-dependence emerged, where the host cell provided a protected environment and nutrients, while the engulfed bacteria contributed essential metabolic functions, notably ATP production. These similarities not only reinforce the endosymbiotic theory but also highlight the transformation of these once-independent entities into complex, interdependent systems, enabling the evolution of more advanced life forms and underscoring the significance of symbiotic relationships in biological diversity .
| Characteristic | Mitochondria | Bacteria |
| Size | 0.5 to 10 micrometers | 0.2 to 10 micrometers |
| DNA Structure | Circular DNA | Circular DNA |
| Ribosomes | 70S ribosomes | 70S ribosomes |
| Reproduction | Binary fission | Binary fission |
| Double Membrane | Present | Present in some (e.g., Gram-negative) |
| Function in Energy Production | ATP production through oxidative phosphorylation | ATP production through various processes (including fermentation and respiration) |
Structural and Functional Similarities Between Mitochondria and Bacteria
A. Double membrane structure and its implications
The intricate relationship between eukaryotic cells and their organelles, particularly mitochondria, underscores a significant evolutionary milestone characterized by mutual dependence. Mitochondria possess a double membrane structure, a feature analogous to that of bacteria, which serves as a compelling piece of evidence for the endosymbiotic theory. This theory posits that ancestral eukaryotic cells engulfed prokaryotic organisms, specifically α-proteobacteria, that eventually transformed into mitochondria. Both mitochondria and their prokaryotic relatives not only share similar biochemical mechanisms, such as ATP production, but also contain their own circular DNA, reinforcing their bacterial ancestry. Over time, this relationship evolved from an initial phase of existence as free-living bacteria to a state of indispensable partnership, wherein eukaryotic cells rely on mitochondria for energy production while mitochondria benefit from the hosts cellular environment for maintenance and replication (Archibald et al.). Thus, the double membrane structure of mitochondria is emblematic of their transformative evolutionary journey.
B. Presence of circular DNA in mitochondria
The presence of circular DNA in mitochondria serves as a compelling piece of evidence supporting the endosymbiotic theory, which posits that these organelles originated from free-living prokaryotic bacteria. Both mitochondria and their bacterial ancestors share several critical similarities, such as the presence of double membranes and the organization of their genomes into circular DNA, akin to that found in bacteria (). This structural resemblance not only suggests a shared ancestry but also points to an evolutionary relationship characterized by mutual dependence; while eukaryotic cells rely on mitochondria for ATP production through aerobic respiration, mitochondria have become reliant on the hosts cellular machinery for their replication and maintenance. Over time, this interdependence has shaped the functioning of eukaryotic cells, illustrating a remarkable evolutionary adaptation that has allowed complex multicellular organisms to thrive ((Guenther Witzany)). Thus, the circular DNA within mitochondria embodies both a genetic legacy and a vital functional partnership in the evolutionary narrative of life.
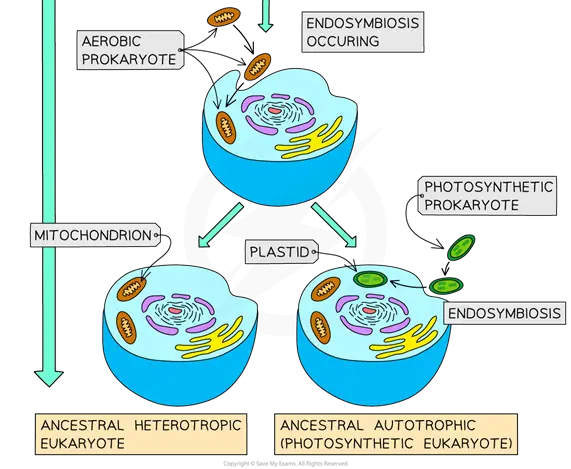
Image: Illustration of the Endosymbiotic Theory in Eukaryotic Evolution
C. Ribosomal similarities and protein synthesis mechanisms
The complexity of eukaryotic life owes much to a significant evolutionary event: the endosymbiotic relationship between ancestral eukaryotic cells and prokaryotic bacteria. Mitochondria, which are crucial for aerobic respiration, share striking similarities with their bacterial ancestors, most notably their double membranes and the presence of circular DNA akin to that found in prokaryotes. This resemblance substantiates the endosymbiotic theory, suggesting that mitochondria originated from engulfed alpha-proteobacteria. Ribosomal similarities further underscore this connection, as mitochondria possess their own ribosomes that resemble bacterial ribosomes rather than those found in eukaryotic cytoplasm. This distinct ribosomal structure facilitates the synthesis of proteins essential for mitochondrial function, establishing a symbiotic dynamic where both the host and the endosymbiont have evolved into a mutually dependent relationship, whereby the eukaryotic cell provides protection and a stable environment, while mitochondria supply vital energy for cellular processes (Claudiu I Bandea), (Archibald et al.).
IV. Evolution of Mutual Dependence Between Host Cells and Mitochondria
The intricate relationship between host cells and mitochondria has been sculpted through eons of evolution, culminating in a profound mutual dependence that is pivotal for both partners. This dynamic originated from an ancient endosymbiotic event, where a primitive eukaryotic host internalized an aerobic prokaryote, which ultimately evolved into the modern mitochondrion. Notably, mitochondria retain distinct similarities to bacteria, most prominently their double membranes and the presence of their own circular DNA, which supports the endosymbiotic theory by underscoring their prokaryotic ancestry. Over time, the association between these two entities transitioned from a situation where the engulfed bacteria might have been parasitic to a stable coexistence where each derives essential benefits—host cells gain ATP through cellular respiration, while mitochondria receive protection and nutrients. This intricate and co-evolutionary process highlights the critical nature of their interdependence, which has become essential for the survival of eukaryotic life (Boza et al.) (Szathmáry et al.).
| Time Period (Mya) | Mitochondrial Genome Size (bp) | Host Cell Size (µm) | Dependency Description | Specialization Level |
| 1500 | 16569 | 10 | Independent prokaryotic ancestors | None |
| 1200 | 16569 | 15 | Increased metabolic cooperation | Low |
| 800 | 16569 | 20 | Enhanced ATP production from oxidative phosphorylation | Moderate |
| 500 | 16000 | 25 | Complete reliance of host on mitochondria for energy | High |
| 100 | 15000 | 30 | Mitochondria as essential organelles in eukaryotic cells | Critical |
Mutual Dependence in Host Cells and Mitochondria
A. Initial symbiotic relationship and its advantages
The intricate relationship between early eukaryotic cells and engulfed prokaryotes marks a pivotal moment in evolutionary history, facilitating the rise of complex life forms. Initially, this symbiotic relationship likely stemmed from an opportunistic interaction, whereby the ancestral eukaryote provided a stable environment for the engulfed aerobic bacteria, which in turn delivered critical metabolic advantages through efficient ATP production. Evidence of this relationship is underscored by the structural similarities between mitochondria and bacteria, such as their double membranes and unique circular DNA, reminiscent of prokaryotic genomes ((Boza et al.)). Over time, this dependence transformed into a more intricate mutualism, where both the host and the engulfed bacterial lineage became indispensable to one another’s survival, ultimately leading to the evolution of modern eukaryotic cells. This profound interdependence not only enhanced cellular efficiency but also paved the way for greater diversity in life forms ((Witzany G)), demonstrating the dynamic nature of evolutionary partnerships.
B. Genetic integration and co-evolution of host and mitochondria
In the enigmatic narrative of cellular evolution, the intricate relationship between ancestral prokaryotes and the evolving eukaryotic host marks a pivotal chapter in the endosymbiotic theory. Mitochondria, reminiscent of their bacterial forebears, share striking similarities such as double membranes and circular DNA, which reinforces the hypothesis of their prokaryotic ancestry (Boza et al.). As these organelles were engulfed by primitive eukaryotic cells, a journey of genetic integration was initiated, leading to an evolutionary trajectory defined by mutual dependence. This co-evolutionary process allowed for a sophisticated division of labor; mitochondria became indispensable for ATP production, while the host provided a stable environment and essential metabolites. Over generations, the symbiotic relationship transformed from a potentially exploitative interaction into a mutually beneficial partnership, essential for the survival of complex multicellular life, thus illustrating a remarkable evolutionary transition that not only changed cellular dynamics but also shaped the trajectory of life itself (Witzany G).
C. Impact on cellular metabolism and energy production
The intricate relationship between mitochondria and ancestral prokaryotes can be traced back to the endosymbiotic theory, which posits that mitochondria originated from free-living bacteria that entered into a symbiotic relationship within a host cell. This relationship established an essential co-dependence that dramatically transformed cellular metabolism and energy production in eukaryotic organisms. Mitochondria share striking similarities with bacteria, including a double-membrane structure and their own circular DNA, which implies a common evolutionary lineage. Through this integration, the host cell acquired the ability to perform oxidative phosphorylation efficiently, thus enhancing ATP production and overall energy metabolism. Over time, this mutual dependence has solidified, as eukaryotic cells have come to rely on mitochondria for energy while the organelles have lost much of their autonomy, leading to a system-level organization wherein both parties are optimized for energy production and cellular function, illustrating a profound evolutionary transition in cellular biology.
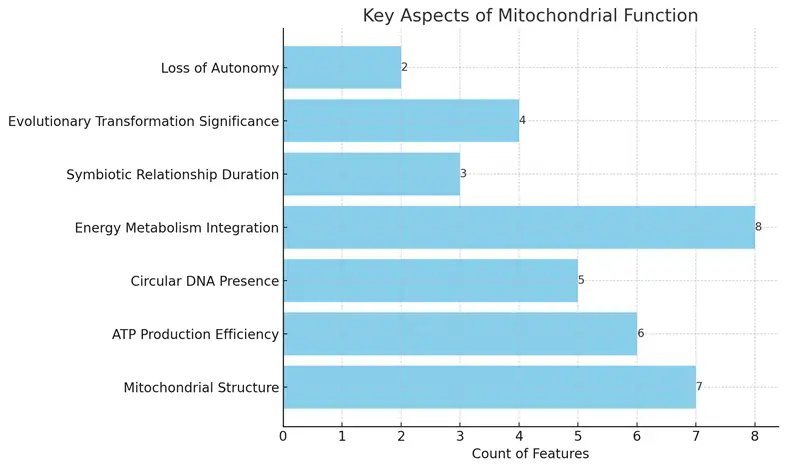
The chart displays the key aspects of mitochondrial function, highlighting their respective counts. Each aspect is represented horizontally, allowing for easy comparison of their significance. The data illustrates features such as mitochondrial structure and ATP production efficiency, which have higher counts, indicating their importance in cellular biology.
V. Conclusion
The intricate relationship between mitochondria and their host cells underscores a remarkable evolutionary journey that reshaped the development of complex life. This partnership originated through an endosymbiotic event where ancestral prokaryotic cells engulfed aerobic bacteria, leading to the establishment of mitochondria—a transformation supported by striking similarities between the two, including their double membranes and circular DNA. Over time, this primary relationship evolved into a mutual dependence, where mitochondria not only provided essential metabolic advantages to eukaryotic cells but also became integral to their survival and functionality. As noted in recent studies, the dual roles of mitochondria extend to not only energy production but also cellular signaling and regulation, highlighting their evolution from independent organisms to indispensable components of cellular machinery (Szathmáry et al.). Consequently, this exemplifies how evolutionary processes can forge complex interdependencies that drive the diversification of life, encapsulating the essence of the endosymbiotic theory as a cornerstone of biological evolution.
A. Summary of key points discussed
The intricate relationship between mitochondria and their ancestral bacterial origins epitomizes a fundamental evolutionary transition, illustrating the principles of the endosymbiotic theory. Central to this theory is the notable similarity between mitochondria and bacteria; both organelles feature double membranes and possess their own circular DNA, resembling that of prokaryotes, which suggests a shared lineage of life forms . This connection underscores the notion that mitochondria once thrived as independent aerobic bacteria before being engulfed by ancestral eukaryotic cells. Over time, this initial interaction evolved into a mutually beneficial arrangement, wherein the host cell provided a protective environment and nutrients, while mitochondria contributed vital energy through cellular respiration, enhancing the overall metabolic efficiency of the host (Szathmáry et al.). Consequently, this symbiotic relationship catalyzed the sophisticated complexity of eukaryotic life, a pivotal milestone in the evolutionary narrative of cellular development.
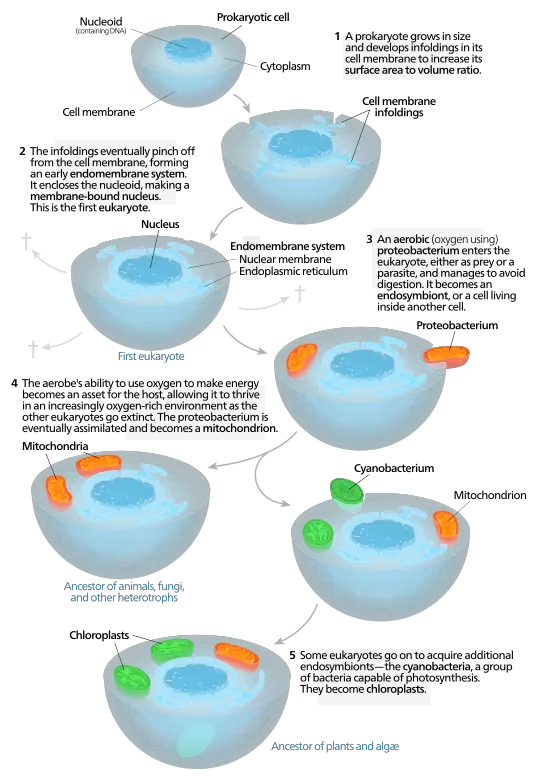
Image: Evolutionary development of eukaryotic cells and organelles.
B. Implications of the endosymbiotic theory for evolutionary biology
The intricate relationships formed through the endosymbiotic theory present profound implications for our understanding of evolutionary biology. Central to this theory is the idea that mitochondria, akin to prokaryotic bacteria, possess double membranes and their own circular DNA, suggesting a shared ancestry that dates back over a billion years. This resemblance supports the notion that ancestral eukaryotic cells engulfed aerobic bacteria, which transitioned into mitochondria, crucial for energy production. Over time, these once-independent entities evolved into essential organelles, reflecting a profound mutual dependence where host organisms rely on mitochondria for ATP generation, while mitochondria benefit from the protective environment and metabolic resources of their hosts. This intricate partnership has not only facilitated the complexity of eukaryotic life forms but has also significantly shaped the evolutionary pathways of diverse organisms, highlighting the interconnectedness of life that persists through symbiotic relationships (Archibald et al.). The evidence is further underscored by studies on horizontal gene transfer, which demonstrate how these interactions have continued to influence genetic diversity and adaptation across taxa (Ham et al.).
C. Future research directions and unanswered questions in the field
The intricate relationship between mitochondria and eukaryotic cells presents intriguing unanswered questions that future research could address, particularly regarding the evolutionary origins of these organelles. The striking similarities between mitochondria and their prokaryotic ancestors—evident in their double membranes, circular DNA, and protein synthesis machinery—underscore the plausibility of their endosymbiotic origin. This mutual dependency, which emerged as eukaryotic cells evolved to utilize aerobic respiration more efficiently, raises pivotal inquiries about the precise mechanisms underlying the transition from autonomy to interdependence. Moreover, exploring how this relationship impacted the evolution of metabolic processes and cellular specialization could offer insights into the evolutionary trajectory of diverse life forms. Investigating these facets not only has the potential to deepen our understanding of mitochondrial function and pathology in modern eukaryotes but also enriches our comprehension of the early evolutionary stages that shaped complex life on Earth.
References
- Claudiu I. Bandea. “A Unifying Scenario on the Origin and Evolution of Cellular and Viral Domains”. 2009, https://core.ac.uk/download/pdf/288874.pdf
- Archibald, John M.. “Endosymbiosis and Eukaryotic Cell Evolution “. Elsevier Ltd., 2015, https://core.ac.uk/download/pdf/82654393.pdf
- Collins, Lesley Joan. “Use of RNA secondary structure for evolutionary relationships : investigating RNase P and RNase MRP : a thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Genetics at Massey University, New Zealand”. ‘Massey University’, 1998, https://core.ac.uk/download/162617286.pdf
- Militello, Guglielmo. “Structural and organisational conditions for the appearance of a functionally integrated organisation in the transition from prokaryotic to eukaryotic cell”. 2021, https://core.ac.uk/download/547398656.pdf
- Szathmáry, Eörs, Zachar, István. “Breath-giving cooperation: critical review of origin of mitochondria hypotheses Major unanswered questions point to the importance of early ecology”. ‘Springer Science and Business Media LLC’, 2017, https://core.ac.uk/download/132277377.pdf
- Beckley, Colin, Bonillas, Ute. “The Evolution of Diversity”. 2017, https://core.ac.uk/download/131214501.pdf
- Guenther Witzany. “Bio-Communication of Bacteria and its Evolutionary Interrelations to Natural Genome Editing Competences of Viruses”. 2008, https://core.ac.uk/download/pdf/287751.pdf
- Boza, G., Zachar, I.. “Endosymbiosis before eukaryotes: mitochondrial establishment in protoeukaryotes”. ‘Springer Science and Business Media LLC’, 2020, https://core.ac.uk/download/287794143.pdf
- Jianping, Du. “Mutation of mitochondria genome: trigger of somatic cell transforming to cancer cell”. BioMed Central, 2010, https://core.ac.uk/download/pdf/8382991.pdf
- Ham, R.C.H.J., van, Latorre, A., Martinez-Torres, D., et al.. “Molecular evolution of aphids and their primary ( Buchnera sp.) and secondary endosymbionts: implications for the role of symbiosis in insect evolution.”. 2001, https://core.ac.uk/download/pdf/29301278.pdf
- Leister, Dario Michael. “Towards understanding the evolution and functional diversification of DNA-containing plant organelles:[version 1; referees: 3 approved]”. ‘F1000 Research Ltd’, 2016, https://core.ac.uk/download/269290630.pdf
- A. Howard, and S.R. Pelc, A.M. van der Bliek, D. Bramhill, H. Ris and W. Plaut, J. Seckbach, K. Nishida, et al.. “Mechanisms of organelle division and inheritance and their implications regarding the origin of eukaryotic cells”. The Japan Academy, 2010, https://core.ac.uk/download/pdf/8574746.pdf
- Duhita Narendra, Hamada Kazuo, Horiike Tokumasa, Miyata Daisuke, Saruhashi Satoshi, Shinozawa Takao. “A Study on the Origin of Peroxisomes: Possibility of Actinobacteria Symbiosis”. 2008, https://core.ac.uk/download/pdf/287814.pdf
- Colnaghi, Marco. “The impact of deleterious mutations on the transition to meiotic sex and the structure of the germline”. UCL (University College London), 2022, https://core.ac.uk/download/519718404.pdf
- Katz, Laura A., Parfrey, Laura Wegener, Tekle, Yonas I.. “Molecular Data are Transforming Hypotheses on the Origin and Diversification of Eukaryotes”. Smith ScholarWorks, 2009, https://core.ac.uk/download/426977271.pdf
