The Golgi Apparatus: A Hub for Protein Sorting and Post-Translational Modifications
I. Introduction
The Golgi apparatus, called the cell’s shipping center, has an important job in processing and sorting proteins made in the endoplasmic reticulum (ER). This organelle is made up of flat sacs, or cisternae, that help change proteins through various modifications, like glycosylation and phosphorylation. These changes are important for how proteins work, affecting their stability, activity, and where they go in the cell. As proteins move through the Golgi apparatus, they are packaged into different vesicles, which are labeled for specific destinations, such as lysosomes, the plasma membrane, or outside the cell. Therefore, it is important to understand the processes happening in the Golgi, as it shows how essential it is for keeping the cell stable and managing the careful distribution of proteins that support many biological functions and pathways.
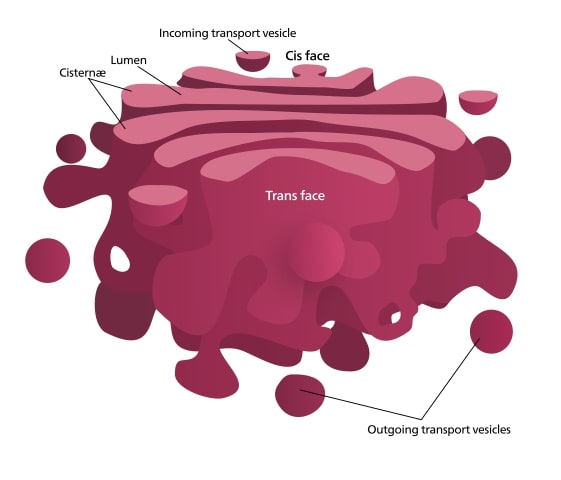
A. Definition and significance of the Golgi apparatus
The Golgi apparatus is an important part of eukaryotic cells, acting as the main center for sorting proteins and making changes after translation. It consists of a series of flattened sacs called cisternae, which work in a sequence to receive, process, and send out proteins that were made in the rough endoplasmic reticulum. As proteins pass through the Golgi, they go through important changes, like adding sugars and phosphate groups, which are necessary for their maturity and correct targeting in the cell (Da Ros et al.). Additionally, the Golgi apparatus is crucial for keeping cellular balance by managing the movement of proteins and lipids to different locations, such as the plasma membrane and lysosomes (Nair et al.). Therefore, the Golgi apparatus not only helps with the last steps of protein development but also manages the complicated interactions needed for cell organization and signaling, highlighting its importance in cell biology.
B. Overview of protein sorting and post-translational modifications
In eukaryotic cells, the Golgi apparatus is important for changing and sorting proteins after they are made. It acts as a central point for these complex tasks. Once proteins are produced in the rough endoplasmic reticulum, they move to the Golgi, where they undergo various changes, like glycosylation and phosphorylation. These changes are key for deciding where a protein goes and how it works. For example, connexins, which help cells communicate, are controlled at both the transcriptional and post-translational levels, with changes influencing their movement and stability in tissues (Aasen et al.). Also, new findings about small RNAs have shown how they contribute to RNA silencing by working with the endomembrane system, indicating that the Golgi may be part of wider regulatory networks (Chen et al.). Therefore, the Golgi apparatus not only helps sort proteins but also links various communication methods in cells that are crucial for balance.
C. Purpose and scope of the essay
This essay aims to explain the important role of the Golgi apparatus in sorting proteins and making changes after translation, which are key to how cells work and are regulated. The Golgi apparatus serves as a main center where proteins made by the cell are modified before they are sent to their right places. These changes, like adding sugars and phosphate groups, are necessary for proteins to function properly and stay stable, which affects how we use them in biotechnology and medicine, as noted in (Saghaleyni et al.). Additionally, this essay will look at how trafficking pathways work with different PDZ domain-containing proteins that affect signaling in receptors, providing information on possible drug targets for mental health issues as shown in (Hammad et al.). By investigating these related topics, this essay seeks to provide a clear understanding of how the Golgi apparatus manages the complex processes of protein development and distribution in the cell.
II. Structure and Function of the Golgi Apparatus
The Golgi apparatus is very important for modifying and sorting proteins made in the endoplasmic reticulum. It has many membrane-bound sacs that help process proteins in different areas. As proteins move from the cis side to the trans side of the Golgi, they go through important changes like glycosylation, which is necessary for their function and how they interact with cells. For example, the GLUT4 protein is carefully controlled during its movement from the Golgi, and the changes it undergoes after translation are crucial for insulin sensitivity and how glucose is taken in by cells (Bayer et al.). Moreover, the Golgi also plays a key role in making different surface glycoproteins, as observed in African trypanosomes, which shows its various roles in protein processing and avoiding the immune system (Adung’a et al.). Therefore, the Golgi apparatus illustrates the complicated processes in cells that are necessary for proper protein handling.
A. Description of the Golgi apparatus architecture
The Golgi apparatus, which has stacked layers of membranes called cisternae, is very important for handling proteins made in the rough endoplasmic reticulum. This organelle has different parts: the cis, medial, and trans Golgi networks, each serving specific roles in adding sugar groups to proteins and modifying them. Within this structure, proteins move between these areas using vesicular transport, which helps in their processing and sending them to where they need to go. Membrane actions are important because proteins go through various changes after they are made, like phosphorylation and ubiquitination, which are necessary for them to work correctly and for communication in cells (Adung’a et al.), (Aasen et al.). The detailed structure of the Golgi apparatus shows how critical it is for sorting proteins and also emphasizes the complex features that help maintain balance in the cell and its reaction to changes in the environment.
B. Role of the Golgi in the endomembrane system
The Golgi apparatus is important in the endomembrane system, managing tasks like protein sorting and post-translational modifications that are vital for how cells work. The Golgi acts as a main center, changing proteins from the endoplasmic reticulum through processes like glycosylation and phosphorylation, helping them to mature and function correctly. Recent studies point out possible links between small RNA activities and the endomembrane system, implying that the Golgi might also be involved in controlling post-transcriptional gene expression with these molecules (Chen et al.). Furthermore, the Golgi plays a key role in moving vacuolar sorting receptors (VSRs), which meet their ligands in the Golgi compartments before being sent to the lytic vacuole through endocytosis, highlighting its essential role in membrane protein management (Fäßler et al.). Thus, the various roles of the Golgi enhance its importance as a central organizer in the endomembrane system.
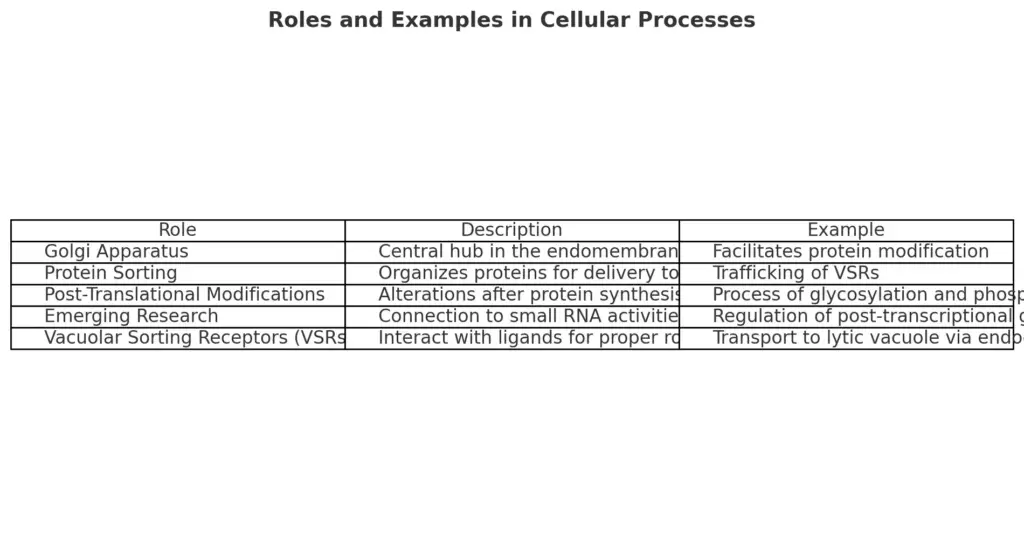
The chart displays various cellular processes, detailing their roles, descriptions, and examples. It organizes information related to the Golgi Apparatus, Protein Sorting, Post-Translational Modifications, Emerging Research, and Vacuolar Sorting Receptors (VSRs), providing clarity on how these components function within a cellular context.
C. Mechanisms of transport within the Golgi apparatus
The Golgi apparatus plays an important role in sorting proteins and modifying them after translation, using specific transport processes that are vital for its function. This organelle gets proteins from the rough endoplasmic reticulum (ER) via vesicular transport, where COPII-coated vesicles help buds and move new proteins towards the Golgi. When proteins enter the Golgi, they go through different modifications, such as glycosylation and phosphorylation, which are important for their function and stability. The movement of proteins within the Golgi is also shaped by vesicular traffic, with COPI vesicles carrying proteins back to the ER to help recycle Golgi enzymes necessary for processing (Adung’a et al.). Additionally, connexins, which are involved in intercellular communication through gap junctions, highlight the various functions of proteins in the Golgi, showing a complex system that regulates protein transport and their post-translational modifications (Aasen et al.).
III. Protein Sorting Mechanisms
The Golgi apparatus is very important for protein sorting, which is needed for keeping cells working properly. When proteins made in the endoplasmic reticulum reach the Golgi, they go through important changes after they are made that decide where they will go. These changes, like adding phosphate groups or sugar chains, are important not just for the protein’s structure but also as signals that direct proteins to the right spots inside or outside the cell. For example, adding sialic acid to proteins makes them more stable and affects how they are transported, as shown by recent studies on the role of variant surface glycoproteins in escaping the immune system (Adung’a et al.). Additionally, the varied functions of connexins show how complex protein movement is, where both gene expression and post-translational modifications significantly influence connexin activity in both healthy and sick states (Aasen et al.). Therefore, the Golgi is a key place where the processes of protein sorting are carefully managed.
A. Types of proteins processed by the Golgi apparatus
The Golgi apparatus is very important for the processing of different kinds of proteins after they are made, especially those that are meant to be secreted or added to the cell membrane. Its complex role can be seen in the various proteins that are modified here, such as integral membrane proteins, secreted proteins, and glycoproteins. For example, connexins, which help cells communicate through gap junctions, are heavily processed in the Golgi. Their phosphorylation changes how they are transported and their stability, affecting how well they function in both health and disease (Aasen et al.). Moreover, proteins like the mouse mammary tumor virus glycoproteins also go through essential changes that control where they go and how they mature, showing the Golgi’s role in important sorting processes needed for their function (APONTE et al.). These actions highlight the Golgi apparatus as a key center for refining proteins and making sure they are correctly distributed within the cell.
| Protein Type | Function | Examples | Post-Translational Modifications |
| Glycoproteins | Cell signaling, immunity, and structure | Antibodies, Hormones | Glycosylation |
| Lipoproteins | Transport of lipids in blood | Cholesterol transport proteins | Lipidation |
| Secretory Proteins | Secretion of proteins outside the cell | Digestive enzymes, Hormones | Phosphorylation, Glycosylation |
| Membrane Proteins | Structural and functional roles in membranes | Receptors, Channels | Palmitoylation, Glycosylation |
| Extracellular Matrix Proteins | Support and anchorage for tissues | Collagen, Fibronectin | Glycosylation, Cross-linking |
Types of Proteins Processed by the Golgi Apparatus
B. Signal sequences and their role in protein sorting
Signal sequences are very important for protein sorting in cells, especially when proteins go through paths to the Golgi apparatus. These hydrophobic peptide sequences are usually found at the beginning of new polypeptides and serve as address labels to guide proteins to the right places, making sure they get to the Golgi for changes and distribution. The detection of these sequences by cell machinery, like signal recognition particles, helps move proteins into the endoplasmic reticulum, which then sends them to the Golgi in COPII-coated vesicles. Moreover, modifications after translation that happen in the Golgi, such as glycosylation and phosphorylation, are affected by the proteins’ first sorting caused by their signal sequences, adding to their functional variety and communication within cells. Therefore, the relationship between signal sequences and protein transport showcases the accuracy of intracellular sorting mechanisms that are vital for cell balance and function (Adung’a et al.), (Aasen et al.).
C. Pathways of protein trafficking from the Golgi to other cellular destinations
The Golgi apparatus is very important for modifying and sorting proteins after they are made, which are sent to different places in the cell. At the trans-Golgi network (TGN), proteins are sorted based on their chemical features and changes, like glycosylation, which affects what happens to them in the cell. Several methods, such as the role of Ca2+-binding proteins like Cab45, are key for moving soluble proteins from the TGN properly. Research has shown that Cab45 helps gather the client proteins in the TGN, which then gets them ready to be packed into secretory vesicles. Additionally, connections with transmembrane proteins such as TGN46 are crucial for sorting, highlighting the complex interactions of molecules that manage protein movement ((Tran et al.)). These processes show how essential the Golgi is as a main center for protein transport, which directly affects the balance and function of the cell ((Munro et al.)).
| Pathway | Destination | Function | Example Protein | Regulation |
| Secretory Pathway | Plasma Membrane | Incorporation of proteins into the plasma membrane. | Insulin | Hormonal signals regulate secretion. |
| Endocytic Pathway | Lysosomes | Delivery of enzymes for degradation. | Lysosomal hydrolases | Receptor-mediated endocytosis. |
| Retrieval Pathway | ER (Endoplasmic Reticulum) | Recycling of ER-resident proteins. | KDEL-tagged proteins | Signal recognition by KDEL receptor. |
| Non-Secretory Pathway | Vesicles for Exocytosis | Transport of proteins to be exocytosed. | Neurotransmitters | Nerve impulses trigger release. |
Protein Trafficking Pathways from the Golgi Apparatus
IV. Post-Translational Modifications
The Golgi apparatus is very important for changing proteins after they are made, which affects how they work and their biological roles. Proteins coming from the endoplasmic reticulum go through several changes, like glycosylation, phosphorylation, and ubiquitination, which are necessary for sorting and moving them around. For example, GLUT4, an important glucose transporter, gets many modifications that help it move to the plasma membrane when insulin is present, showing how these changes connect to cellular signaling (Bayer et al.). Moreover, African trypanosomes illustrate how these modifications, especially in their variant surface glycoproteins (VSGs), help them avoid immune responses, showing the various functions that come from these changes (Adung’a et al.). Therefore, the Golgi apparatus acts not only as a place for sorting but also as a key player in the regulatory processes that influence how cells behave through post-translational modifications.
A. Overview of common post-translational modifications (PTMs)
Post-translational modifications (PTMs) are important steps that increase what proteins can do in the Golgi apparatus, affecting how they work and how stable they are. Some common PTMs include phosphorylation, glycosylation, ubiquitination, and methylation, each playing its own role in controlling protein function. Phosphorylation usually changes the activity of enzymes and how proteins interact with each other, while glycosylation is crucial for protein folding and stability, significantly impacting cell signaling and immune functions. Ubiquitination impacts how proteins are broken down and is key to maintaining quality control in cells. Moreover, methylation can affect how proteins interact with one another and has a part in regulating genes. Because the Golgi plays a key role in processing these modifications, understanding the dynamics of PTMs is essential for exploring their effects on health and disease, especially in relation to protein secretion pathways and the difficulties faced in biotechnology and therapeutic development (Saghaleyni et al.), (Cai et al.).
| Modification | Function | Enzymes Involved | Commonly Modified Residues |
| Phosphorylation | Regulates enzyme activity and protein interactions | Kinases, Phosphatases | Serine, Threonine, Tyrosine |
| Glycosylation | Affects protein folding, stability, and cell signaling | Glycosyltransferases | Asparagine, Serine, Threonine |
| Ubiquitination | Targets proteins for degradation or alters activity | Ubiquitin ligases, Deubiquitinating enzymes | Lysine |
| Methylation | Regulates gene expression and protein interactions | Methyltransferases, Demethylases | Lysine, Arginine |
| Acetylation | Regulates gene expression and protein stability | Acetyltransferases, Deacetylases | Lysine |
Common Post-Translational Modifications
B. The role of the Golgi in glycosylation processes
The Golgi apparatus is very important for glycosylation, which is how proteins are processed and sorted. It shapes the sugar structures that are added to new proteins. When glycoproteins go through the Golgi’s layers, they undergo changes, adding sugars that influence how stable, where they go, and how they interact with other molecules. This modification after proteins are made is vital for their proper work, especially for proteins that help pathogens avoid the immune system, like variant surface glycoproteins (VSGs) found in Trypanosoma brucei (Adung’a et al.). The Golgi apparatus controls a complex array of enzymes that help these glycosylation reactions, connecting metabolic pathways with how proteins are transported (A Engling et al.). This detailed function emphasizes the Golgi’s role not just in sorting proteins but also in making sure glycosylation is accurate, which is essential for cell signaling and the immune response.
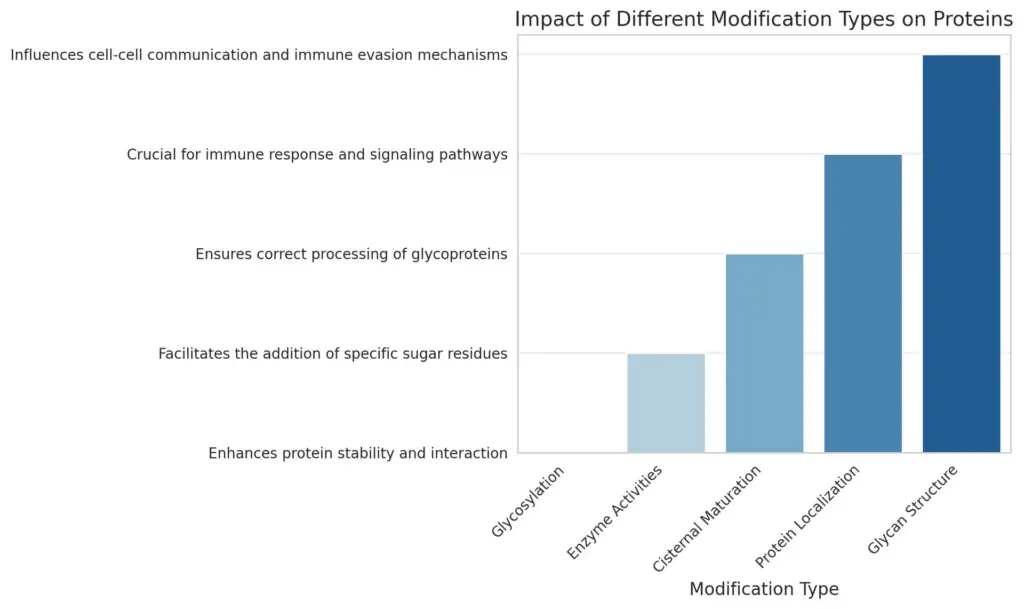
The chart displays the impact of various protein modification types, highlighting their significance in biological processes. Each bar represents a different modification type, with corresponding impacts listed on the y-axis, conveying essential roles in protein functionality, stability, and interaction within the cellular environment.
C. Impact of PTMs on protein function and cellular signaling
The effects of post-translational modifications (PTMs) on how proteins work and signal in cells are very important, especially as proteins move through the complex Golgi apparatus. When proteins experience changes like phosphorylation, glycosylation, and ubiquitination, their shape and function can change a lot, affecting different signaling pathways in the cell. For example, phosphorylation can affect how stable and where proteins are located, which is vital for cellular communication and balance (Aasen et al.). Also, new findings show that small RNAs, like miRNAs and siRNAs, play a role in controlling gene expression and protein function, further indicating a complex level of control in the endomembrane system (Chen et al.). These modifications and regulatory interactions show that the Golgi apparatus is not only a processing area but also an important part of managing complicated networks that control cellular signaling and functions.
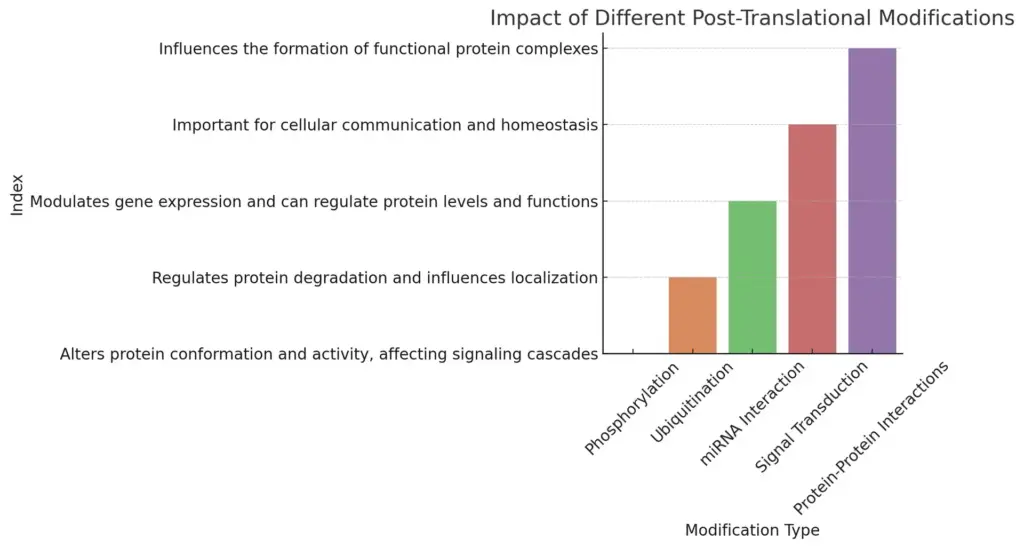
The chart illustrates the impact of various post-translational modifications (PTMs) including phosphorylation, ubiquitination, miRNA interaction, signal transduction, and protein-protein interactions. Each modification is associated with a unique process and significantly influences cellular functions, communication, and protein stability.
V. Conclusion
To wrap up, the Golgi apparatus is an essential center for sorting proteins and making modifications after translation, highlighting its key part in cell function. The complex actions required for moving proteins from the endoplasmic reticulum to the Golgi are mainly carried out by COPII and COPI systems, which adjust to different types of cargo and the needs of the cell, as shown by the specific export of glycosylphosphatidylinositol (GPI)-anchored proteins (López Martín et al.). Moreover, new findings show links between the Golgi and small RNA-related RNA silencing, suggesting the endomembrane system has a more important role in regulating gene expression after transcription than previously thought (Chen et al.). Together, these revelations stress the many roles of the Golgi, not just in processing and sorting proteins, but also in broader RNA regulation, thus improving understanding of cellular balance and signaling pathways essential for life.
A. Summary of the Golgi apparatus’s roles in protein sorting and modifications
The Golgi apparatus is very important as a site for processing and sorting proteins, supporting key cellular activities. New proteins that come from the rough endoplasmic reticulum go through important post-translational changes like glycosylation and phosphorylation as they move through the Golgi’s stacked structures. These changes are necessary for proteins to fold correctly and stay stable, and any alterations can affect how they function and where they go in the cell. For example, GLUT4, a protein important for managing glucose levels, experiences significant modifications in the Golgi that help control its movement to the plasma membrane when insulin is present, which shows how this organelle impacts metabolic signaling pathways (Bayer et al.). Moreover, proteins like connexins have modifications that affect their stability, function, and ability to communicate between cells, demonstrating the Golgi’s diverse roles in keeping cellular balance and responding to physiological changes (Aasen et al.). Therefore, the Golgi apparatus is a crucial center for managing protein actions and cellular activities.
B. Implications for cellular function and health
The Golgi apparatus is very important for keeping cells working well and staying healthy. It does this by sorting proteins and helping with key post-translational modifications. These modifications are important because they manage how proteins work and affect how cells communicate with each other. For example, problems with protein modifications can lead to faulty connexins, which are needed for communication between cells and maintaining tissue balance. Such problems can lead to various health issues, including developmental disorders and tumors, showing how important it is for the Golgi to function well to avoid diseases (Aasen et al.). In addition, new studies show that the Golgi apparatus is linked to small RNA processes that control gene activity, which reveals a less noticed part of its job in gene silencing and responses to stress in cells (Chen et al.). All of this indicates that the health of the Golgi apparatus is crucial for keeping cells balanced and preventing illnesses.
C. Future research directions and potential therapeutic applications
As studies keep showing the many roles of the Golgi apparatus in sorting proteins and making changes after translation, future research will probably focus on finding medical uses for these processes. One hopeful area is changing Golgi-related glycosylation patterns to make biologic drugs, like monoclonal antibodies, work better by increasing their stability and effectiveness. Additionally, knowing how the Golgi contributes to diseases such as cancer and nerve disorders might help create new biomarkers and focused treatments. Using advanced imaging methods and molecular biology tools could help us see how Golgi functions interact with cell signaling pathways, leading to new discoveries in regenerative medicine and drug delivery methods. Improving our understanding of the Golgi apparatus not only boosts basic biological science but also paves the way for new therapies to tackle major health issues.
REFERENCES
- Adung’a, Ali, Allen, Anitei, Banerjee, Bangs, Bangs, et al.. “Life and times:synthesis, trafficking, and evolution of VSG”. ‘Elsevier BV’, 2014, https://core.ac.uk/download/30661565.pdf
- Aasen, Trond, Johnstone, Scott, Koval, Michael, Lynn, et al.. “Connexins: synthesis, post-translational modifications, and trafficking in health and disease”. ‘MDPI AG’, 2018, https://core.ac.uk/download/157862772.pdf
- Bayer, Begum, Birnbaum, Bogan, Bogan, Bogan, Bryant, et al.. “Posttranslational modifications of GLUT4 affect its subcellular localization and translocation”. ‘MDPI AG’, 2013, https://core.ac.uk/download/16459192.pdf
- APONTE, Gregory W., Bravo, D, Firestone, G, Haffar, et al.. “Glucocorticoid-regulated localization of cell surface glycoproteins in rat hepatoma cells is mediated within the Golgi complex.”. eScholarship, University of California, 1988, https://core.ac.uk/download/323082237.pdf
- Chen, Xuemei, Kim, Yun Ju, Maizel, Alexis. “Traffic into silence: endomembranes and post-transcriptional RNA silencing.”. eScholarship, University of California, 2014, https://core.ac.uk/download/323067964.pdf
- López Martín, Sergio, Muñiz Guinea, Manuel, Rodríguez Gallardo, Sofía, Sabido Bozo, et al.. “Endoplasmic Reticulum Export of GPI-Anchored Proteins”. ‘MDPI AG’, 2019, https://core.ac.uk/download/227043802.pdf
- A Engling, A Idiris, AA McCracken, AC Horton, AJ Parodi, Amir Feizi, AS Robinson, et al.. “Genome-scale modeling of the protein secretory machinery in yeast”. ‘Public Library of Science (PLoS)’, 2013, https://core.ac.uk/download/18493919.pdf
- Saghaleyni, Rasool. “Systems Biology of Protein Secretion in Human Cells: Multi-omics Analysis and Modeling of the Protein Secretion Process in Human Cells and its Application.”. 2021, https://core.ac.uk/download/475664119.pdf
- Cai, Siqi, Ding, Siyuan, Li, Li, Li, et al.. “When STING meets viruses: Sensing, trafficking and response”. Digital Commons@Becker, 2020, https://core.ac.uk/download/343747784.pdf
- Fäßler, Florian. “In vivo analysis of endocytic and biosynthetic transport to the plant vacuole”. Universität Tübingen, 2018, https://core.ac.uk/download/160236502.pdf
- Tran, Mai Ly. “Mechanisms of secretory cargo sorting at the trans-Golgi network”. Ludwig-Maximilians-Universität München, 2022, https://core.ac.uk/download/511395706.pdf
- Munro, Sean. “What is the Golgi apparatus, and why are we asking?”. 2011, https://core.ac.uk/download/42335976.pdf
- Hammad, Maha Mahmoud. “CAL and MAGI PDZ Protein Regulation of CRFR1 and 5-HT2AR Trafficking and Signaling”. Scholarship@Western, 2016, https://core.ac.uk/download/61691013.pdf
- Da Ros, Matteo. “The Chromatoid body entourage: Molecular characterization of the Chromatoid body‐associated cytoplasmic vesicles”. fi=Turun yliopisto|en=University of Turku|, 2015, https://core.ac.uk/download/198000428.pdf
- Nair, Prashant. “Signals involved in protein intracellular sorting”. 2005, https://core.ac.uk/download/18233501.pdf
